What molecule am I?
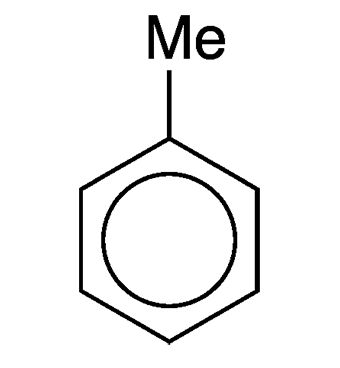
March is MOTW Solvent Month! This is the third of four articles about key solvents—Ed.
Toluene, or toluol as it was once called, is the simplest aromatic hydrocarbon except for benzene. It was first isolated in 1837 from pine oil by Polish chemist Filip N. Walter. Four years later, French chemist Henri Étienne Sainte-Claire Deville isolated it from “tolu balsam” (extracted from the Colombian tree Myroxylon balsamum). Within the next 10 years, chemists settled on toluene as the name for the then-new substance.
As shown in the statistics table, almost all of the global toluene demand is produced in oil refineries or is a byproduct of ethylene crackers. Similarly, most of it is used to make other aromatics or as a gasoline additive. Its use as a solvent is relatively minor; but it is a primary ingredient in paints, lacquers, and resins.
Toluene has largely replaced the more-toxic benzene as a solvent. Benzene is an established carcinogen, but the carcinogenicity of toluene is as yet undetermined.
Toluene hazard information
| GHS classification*: Flammable liquids, category 2 | |
| H225—Highly flammable liquid and vapor | |
| GHS classification: aspiration hazard, category 1 | |
| H304—May be fatal if swallowed and enters airways | |
| GHS classification: skin irritation, category 2 | |
| H315—Causes skin irritation | |
| GHS classification: specific target organ toxicity, single exposure, central nervous system, category 3 | |
| H336—May cause drowsiness or dizziness | |
| GHS classification: reproductive toxicity, category 2 | |
| H361—Suspected of damaging fertility or the unborn child | |
| GHS classification: specific target organ toxicity, repeated exposure, category 2 | |
| H373—May cause damage to organs through prolonged or repeated exposure | |
| GHS classification: acute aquatic toxicity, category 2 | |
| H401—Toxic to aquatic life | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Toluene fast facts
| CAS Reg. No. | 108-88-3 |
| Empirical formula | C7H8 |
| Molar mass | 92.14 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 111 ºC |
| Water solubility | 526 mg/L |
Toluene statistics
| Global demand (2018) | ≈27 million tonnes |
| Production | |
| From oil refineries | 75% |
| From ethylene steam crackers | 20% |
| Styrene byproduct, coke oven light oil | 5% |
| Usage | |
| Interconversion to benzene and xylenes | 50% |
| Blending into gasoline to raise octane number | 25% |
| Solvent | 18% |
| Toluene diisocyanate feedstock | 5% |
| Benzoic acid feedstock | 2% |
Courtesy IHS Markit Ltd.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

