What molecule am I?
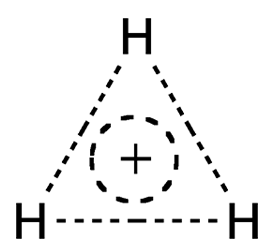
Like the helium hydride cation (HeH+), the Molecule of the Week for May 20, 2019, the trihydrogen cation (H3+) looks as though it shouldn’t exist. Also like HeH+, H3+ was discovered a long time ago and is abundant in outer space.
In 1911, the British physicist and 1906 Nobel Prize winner J. J. Thomson observed the presence of a molecular ion with a mass/charge ratio of 3:1 in the discharge from a plasma tube. He deduced that the only possible formula for the ion could be H3+.
Fifty years later, D. W. Martin, E. W. McDaniel, and M. L. Meeks at Georgia Tech (Atlanta) speculated the existence of H3+ in space. Now, almost 30 years later, relying on a prediction of the ion’s spectrum, Pierre Drossart at the Paris Observatory, Steve Miller at University College London, and their colleagues found the elusive ion in the auroras of Jupiter.
H3+ was subsequently found in the atmospheres of Saturn and Uranus as well as Jupiter. It is also plentiful in interstellar space.
Because it exists only at very low pressure and temperature, there are few physical and chemical data and no hazard information available for H3+. But astrochemists and other space scientists find it a convenient tool for examining chemical bonding and reactions in the observable universe.
Trihydrogen cation
fast facts
| CAS Reg. No. | 28132-48-1 |
| Empirical formula | H3 |
| Molar mass | 3.02 g/mol |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

