What molecule am I?
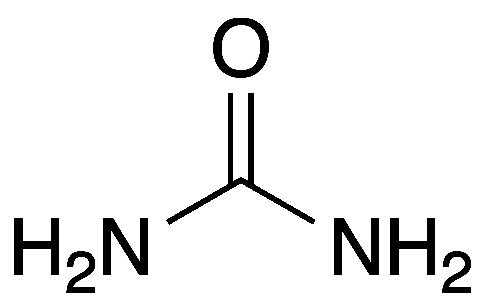
Urea, also known as carbamide, is a safe, useful compound with a significant history. It is a naturally occurring molecule that is produced by protein metabolism and found abundantly in mammalian urine.
In 1828, the German chemist Friedrich Wöhler1, then at the Polytechnic School (now Technical University) of Berlin, published a seminal article in which he demonstrated that a biomolecule, urea, can be synthesized from a nonbiological starting material. Wöhler prepared the inorganic compound ammonium cyanate in the lab, then heated it, causing it to isomerize to urea. Now known as the “Wöhler synthesis”, the reaction helped to disprove the concept of vitalism, which held that “organic” molecules can be made only by living organisms.2
In a reaction similar to the Wöhler synthesis, ammonium carbamate can be converted to urea and water. This is the basis of the process that has been used to produce urea industrially for almost a century. Ammonia and carbon dioxide (CO2) react exothermically to produce the carbamate salt, which is then heated to form urea. The heat produced in the first reaction drives the second. Typically, ammonia and urea are manufactured in the same plant so that some of the carbon dioxide byproduct from ammonia production can be used to make urea.
Global urea production capacity is ≈220 million t/year. Why is urea produced in such large quantities? The answer is that, other than ammonia, urea has the highest nitrogen content of all industrial chemicals and is in high demand as a fertilizer. In the soil, it decomposes back to ammonia (actually ammonium ion) and carbon dioxide. Nitrogen-fixing bacteria oxidize ammonium to nitrate, which is readily taken up by the roots of crops. In addition to its high nitrogen content, urea is particularly useful because it can be applied as a solid in pellet form; and its unusually high solubility in water allows it to be incorporated into solutions with other plant nutrients.
More than 90% of urea production goes into agriculture. The remaining ≈20 million t made annually goes into animal feed (cattle, among others, can convert it into protein), urea–formaldehyde resins, emollients for skin care, and barbituric acid manufacture. Urea’s strongly negative heat of solution in water is the basis of instant-cold packs, in which plastic pouches contain urea and water in separate compartments. When the seal between them is broken, intermixing produces short-term cooling for aching joints and muscles.
There’s always room for improvement. In a 2018 article, British scientific writer David Bradley described ways in which urea might be used more efficiently in agriculture. And last year, in what might be termed a “urea revolution”, Shuangyin Wang and colleagues at Hunan University (Changsha, China) and other institutions described an electrochemical route to urea.
Although urea is used widely in agriculture, current urea production is decidedly not “green”. Ammonia and urea production consume >2% of the world’s energy and emit more CO2 than any other industrial process. Wang’s group developed an electrochemical method that skips ammonia and directly converts nitrogen gas, CO2, and water to urea at ambient temperature and pressure. The synthetic route is complex, and the process is not yet efficient or sufficiently productive, but the objective is certainly well worth striving toward.
1. Wöhler was truly a pioneering chemist. In addition to his urea synthesis, he isolated the elements beryllium and yttrium in pure form, synthesized several then-unknown inorganic compounds, and introduced the concept of organic functional groups
2. After his discovery, Wöhler wrote, “I can no longer hold my chemical water. I must tell you that I can make urea without the use of kidneys of any animal, be it man or dog.”
Urea hazard information
| Hazard class* | Hazard statement |
|---|---|
| Not a hazardous substance or mixture |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Urea fast facts
| CAS Reg. No. | 57-13-6 |
| SciFinder nomenclature | Urea |
| Empirical formula | CH4N2O |
| Molar mass | 60.06 g/mol |
| Appearance | White crystals or powder |
| Melting point | 133–135 ºC |
| Water solubility | 1.1 kg/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

