What molecule am I?
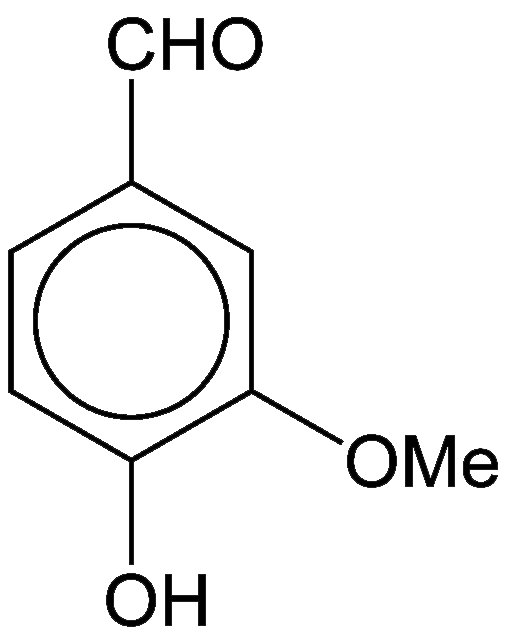
Vanillin, as its name implies, is the major flavor component of vanilla. The three oxygen atoms in this small aromatic compound are in different functional groups: alcohol, aldehyde, and ether. It is a white crystalline solid with a melting point of 81–83 ºC.
The Aztecs used vanilla to flavor chocolate as early as the 16th century, but vanillin was not isolated until 1858, when French biochemist Nicolas-Theodore Gobley crystallized it from vanilla extract. In 1874, German scientists Ferdinand Tiemann and Wilhelm Haarmann determined its structure and synthesized it from coniferin, a component of pine bark.
Flash forward to today: Almost all vanillin used in foods is manufactured, mostly from petrochemical feedstocks. But the food industry is on a tear to label more and more products as consisting entirely of “natural” ingredients. This trend puts the agricultural industry under pressure to genetically modify plants to produce greater amounts of vanillin. To find out how they’re doing it, see Melody Bomgardner’s cover story in the September 12 issue of C&EN.
MOTW update:
November 27, 2023
Vanillin1 was the Molecule of the Week for September 13, 2016. The major flavor component of vanilla, it occurs in nature in vanilla beans (primarily Vanilla planifolia), but almost all commercial vanillin is synthetic. Like trans-cinnamaldehyde, cited above, vanillin was shown this month to have an unexpected biological effect. Shunlin Zheng and collaborators at Sichuan Agricultural University (Chengdu, China) and other Chinese institutions, in a 10-year study, found a negative correlation between potato yield and continuous cropping years. They discovered that the decline in adventitious potato root numbers was primarily the result of soil vanillin accumulation.
1. CAS Reg. No. 121-33-5.
MOTW update:
May 25, 2020
Vanillin is, of course, the major flavor component of vanilla. Most vanillin is made from petrochemical precursors, but now producers are seeking more sustainable sources. Siegfried R. Waldvogel and co-workers at Johannes Gutenberg University Mainz (Germany) report that by using an electrochemical reaction, they can produce vanillin and other useful compounds from lignin, a paper pulp byproduct.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

