What molecule am I?
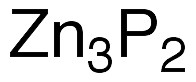
Zinc phosphide (Zn3P2) is a synthetic inorganic compound that has widely divergent uses. It was originally prepared in the early 20th century by heating stoichiometric amounts of the elements. Much more recently (2013), Erik J. Luber*, Md Hosnay Mobarok, and Jillian M. Buriak* at Canada’s National Institute for Nanotechnology and the University of Alberta (both in Edmonton) prepared pure nanocrystalline Zn3P2 by treating tri-n-octylphosphine (TOP) with dimethylzinc at 320 °C.
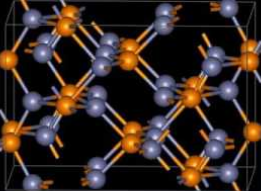
Under ambient conditions, zinc phosphide has a tetragonal crystal structure (shown); it converts to cubic when heated to ≈845 °C. This brings up an unusual situation about Zn3P2’s melting point: Some references report that it melts at 1160 °C or higher, but others give 420 °C. The phase-change temperature clearly indicates that the higher melting point is correct.
The original use for zinc phosphide was as a rodenticide, for burrowing pests such as gophers and moles and for domestic pests such as rats and mice. It is usually combined with baits that the animals ingest. Zn3P2 hydrolyzes slowly in contact with water; but the acidity in the target pest’s stomach rapidly releases highly toxic phosphine gas, a respiratory poison. Phosphine was the Molecule of the Week for October 22, 2018.
The newer use of zinc phosphide is more pleasant and exciting. In 2009, Gregory M. Kimball and co-workers at Caltech (Pasadena, CA) reported that steady-state photoluminescence spectra of Zn3P2 wafers have a fundamental indirect band gap of 1.38 eV, close to the ideal direct band gap value of 1.5 eV. This result indicates that it may be valuable as a semiconductor in photovoltaic cells.
The work of Luber et al., mentioned above, also involved photovoltaics. Their synthetic method produced colloidal semiconducting ≈8-nm nanocrystals with the tetragonal (α-Zn3P2) structure. They found that the optical band gap in this form is 0.5 eV greater than that of bulk Zn3P2. Films prepared via deposition of the nanoparticles were used in heterojunction devices that had excellent rectification behavior. Other data, however, indicated that the particles had phosphorus-rich shells (due to the presence of elemental phosphorus [P(0)]), which hindered performance.
Subsequently, Luber, Buriak, and colleagues developed an alternative method for preparing nanocrystalline zinc phosphide that uses tris(trimethylsilyl)phosphine instead of TOP as the phosphorus source. This change greatly decreased the concentration of P(0) on the particle surfaces.
Zinc phosphide hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Substances and mixtures that, in contact with water, emit flammable gases, category 1 | H260—In contact with water releases flammable gases that may ignite spontaneously | |
| Acute toxicity, oral, category 1 | H300—Fatal if swallowed | |
| Acute toxicity, dermal, category 4 | H312—Harmful in contact with skin | |
| Short-term (acute) aquatic hazard, category 1 | H400—Very toxic to aquatic life | |
| Long-term (chronic) aquatic hazard, category 1 | H410—Very toxic to aquatic life with long-lasting effects | |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Zinc phosphide fast facts
| CAS Reg. No. | 1314-84-7 |
| SciFinder nomenclature | Zinc phosphide |
| Empirical formula | P2Zn3 |
| Molar mass | 285.12 g/mol |
| Appearance | Gray to black crystals or powder |
| Melting point | >1160 °C |
| Water solubility | Reacts |
MOTF update
The Molecule of the Future for June 21, 2021 was GS-441524, the core structure of Gilead Science’s remdesivir. A letter published in Chemical & Engineering News on the same date cites efforts to bring GS-441524 to clinic in 2020. It thus appears that GS-441524 has a past as well as a future.
Over the years, readers have noted that ionic substances are not actually molecules. This is correct, but we use "molecules" in the broadest sense to include them in Molecule of the Week.—Ed.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

