FOR IMMEDIATE RELEASE | August 25, 2021
Evolutionary ‘time travel’ reveals enzyme’s origins, possible future designs
Note to journalists: Please report that this research will be presented at a meeting of the American Chemical Society.
A media briefing on this topic is available at www.acs.org/acsfall2021briefings.
ATLANTA, Aug. 25, 2021 — “The distinction between the past, present and future is only a stubbornly persistent illusion,” Albert Einstein wrote. Perhaps this is nowhere more evident than in protein evolution, where past and present versions of the same enzyme exist in different species today, with implications for future enzyme design. Now, researchers have used evolutionary “time travel” to learn how an enzyme evolved over time, from one of Earth’s most ancient organisms to modern-day humans.
The researchers will present their results today at the fall meeting of the American Chemical Society (ACS). ACS Fall 2021 is a hybrid meeting being held virtually and in-person Aug. 22-26, and on-demand content will be available Aug. 30-Sept. 30. The meeting features more than 7,000 presentations on a wide range of science topics.
“If a person lives in present-day Rome, they might want to learn about ancient Rome to better understand who they are,” says Magnus Wolf-Watz, Ph.D., the project’s principal investigator. “In the same way, we can look backward in time at more ancient forms of enzymes to understand how the proteins are working today and how we might engineer new versions in the future.”
Wolf-Watz, who is at Umeå University in Sweden, looked back some 2 to 3 billion years to primitive organisms known as archaea. These single-celled life forms, which still exist today, have characteristics of both prokaryotes (bacteria, which lack a cell nucleus) and eukaryotes (organisms like plants, animals and fungi that have a nucleus in their cells). A branch of archaea known as the Asgard phylum, discovered in 2015, comprises the closest known ancestors to eukaryotic cells. Four types of Asgard archaea have been identified, including Odin archaea, found in hydrothermal vents deep in the Atlantic Ocean.
Odin archaea have an enzyme called adenylate kinase (AK), which is also found in prokaryotes and eukaryotes. Wolf-Watz previously studied two human types of this enzyme, AK1 and AK3. Both are important in maintaining the energy balance in cells, but AK1 is in the cytoplasm, where it transfers a phosphate group from adenosine triphosphate (ATP, the main energy carrier in cells) to adenosine monophosphate (AMP). In contrast, AK3 resides within mitochondria, where it transfers a phosphate group from guanosine triphosphate (GTP, a molecule similar to ATP but with distinct roles) to AMP.
Wolf-Watz’s team used X-ray crystallization and nuclear magnetic resonance spectroscopy to study the structures of AK1 and AK3, finding that although the enzymes are very similar, they have a subtle difference in a short loop region that causes AK1 to prefer ATP and AK3 to prefer GTP. “Now we can take any AK enzyme, look at the structure of that loop, and predict whether it’s going to use ATP or GTP,” Wolf-Watz says. The next step was to examine a more ancient version of an AK enzyme –– from Odin archaea –– to learn how AK1 and AK3 evolved to prefer different nucleotide substrates.
The researchers purified the archaea AK and determined its structure. They found that the loop important for discriminating ATP and GTP is much longer in the archaeal enzyme, and it has chemical groups that can bind either nucleotide. “What we found is an early ancestor of the human AKs that contains two capacities — it can use both ATP and GTP,” Wolf-Watz says. “During the course of evolution, it became specialized to become specific for one or the other, depending on the cellular compartment where it resides.” The archaea AK can actually use all naturally occurring nucleotide triphosphates (NTPs). “We’ve discovered a universal NTP binding motif that could be a building block for the future design of novel enzymes,” Wolf-Watz says.
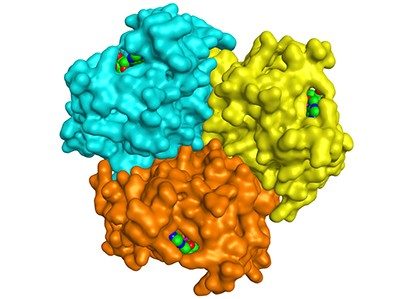
The archaeal AK contains three copies of the enzyme (known as a trimer) that bind to each other through a helical structure. In human AKs, a mutation in this region makes the enzyme copies unable to stick to each other. The human enzymes, which function independently, are almost 1,000-fold more active. The trimer could have been more stable in the extreme environment of hydrothermal vents, but the human enzymes might have traded this thermostability for higher activity, which is important in a cooler environment, Wolf-Watz says.
Next, the researchers want to engineer novel enzymes that could be useful in organic synthesis or drug development. They also want to examine other enzymes from Odin archaea and study how they might have evolved over the eons. “We studied one enzyme and made this fantastic discovery,” Wolf-Watz says. “Of course, there’s more to find. It’s like we’re digging through a treasure chest.”
The researchers acknowledge support and funding from the Swedish Research Council, the Kempe Foundations and the Carl Trygger Foundation.
To automatically receive press releases from the American Chemical Society, contact newsroom@acs.org.
Note: ACS does not conduct research, but publishes and publicizes peer-reviewed scientific studies.
Media Contact
ACS Newsroom
newsroom@acs.org
View larger image

