Radiation: The Good, The Bad, and Its Place in Our Modern World

Amelia “Mollie” Maggia was considered one of the best among hundreds of women factory workers employed in the early 20th century to paint watches. At the end of a long day, Mollie and her female colleagues would leave the factory glowing, covered in powdered radium-containing paint. Branded Undark, the paint glowed a brilliant green. In the late 1910s and early 1920s, the United States Radium Corporation (USRC) used it to create glow-in-the-dark watches, which were popular with soldiers in World War I.
Mollie and her coworkers were trained to shape the bristles of their paintbrushes with their lips. Each time Mollie molded the brush, she ingested a little bit of the paint. What she didn’t know was that a dangerous nuclear reaction was occurring in the paint and also inside of her body. She was one of the first to suffer agonizing pain in her limbs and jaw bone. Within 5 years of exposure, she died from radium poisoning at age 24 in 1922.
Undark had two main ingredients: a fluorescent phosphor that glows a brilliant green when excited and radioactive 226Ra, which excites the phosphor. Illnesses experienced by the “Radium Girls,” as Mollie and her coworkers came to be known, led to historic labor laws that now protect workers from unsafe workplaces.
Although it can be destructive, radioactivity can also be beneficial, especially when it comes to medical tests and certain medical treatments. Not only can radiation find tumors within the body, it can also destroy them or slow their growth. Radiation also makes it possible to study matter, plants, and animals in an effort to understand the universe better.
Read on to learn more about the history of misuses and transcendance of radioactivity to modern applications.

Radioactivity from lab to home
In 1896, about a year after the discovery of X-rays, Henri Becquerel discovered that uranium spontaneously emits a penetrating radiation that can be registered on a photographic plate. Although fluorescence and phosphorescence were known phenomena, they require an external energy source. Uranium was radiating all on its own. His then-student Marie Curie studied the phenomenon for her thesis, coining the term “radioactivity.” She and her husband, Pierre, explored the nature of radioactivity and found it was a nuclear, not chemical, characteristic--the same amount of radiation was emitted by pure elements as by elements in minerals. In 1903, the three scientists shared the Nobel Prize in Physics for their discovery. The Curies continued their study of radioactivity and in 1911 won a Nobel prize for the discovery of the elements radium and polonium and study of their compounds. Fascination with this newfound spontaneous radioactivity spread quickly, both inside the lab and commercially.
Capitalizing on that popularity, manufacturers incorporated radioactive elements into products for their supposed cosmetic and healing properties and because they made things glow. Some products included chocolate, glow-in-the dark toys (and watches!), health tonics, suppositories, and soaking mud for skin. A number of radium and radon-infused spas popped up in the 1920s and 1930s (a few of which are still in existence in the United States, Japan, and Europe), claiming to provide a “healthy” glow. Additionally, brightly colored uranium salts were incorporated into glass and dinner plates until the 1970s, when sales of the radioactive dinnerware dropped.
Where it all begins
Radioactivity originates in atomic nuclei, which are held together by strong nuclear force. Because strong force comes from the quarks that make up the nucleons (protons and neutrons), more nucleons mean more strong force. But protons are positively charged, and so they repel each other. Adding more neutrons adds more strong force without adding charge. Thus, heavy elements such as 197Au are stable despite having a large nucleus.
However, strong force is only effective over short distances (~10-15 m). Very large nuclei become unstable and decay into more stable species, releasing radiation as they do. Every element heavier than lead is inherently unstable—and radioactive—as are certain isotopes of lighter elements. Additionally, many other elements have at least one unstable isotope.
There are certain “magic numbers” of protons and neutrons that appear to be particularly stable, suggesting that nuclei fill in shells, just as electrons do. Nucleons also pair up, so even numbers are more stable than odd. This is why some light elements (think 91Tc or 3H) are also radioactive.
Dangers of radioactivity
Mollie had a toothache. The tooth was removed, but her gums never healed and the pain spread. She had more teeth removed, and painful ulcers arose in their place. In May 1922, her doctor reached into her mouth and, to his surprise, was able to lift out her loose, hole-filled jawbone.
Mollie Maggia was the first dial painter to die from radium poisoning, though she was not the last. Some women had radium jaw, like Mollie. Others developed cancer and giant sarcomas of the bone. And some survived for years but lived with painful locking joints.
The true danger of radium and other radioactive elements is that their nuclei emit one or more types of ionizing radiation when they decay (see sidebar). 226Ra emits alpha particles to become 222Rn, which further decays to 218Po; the alpha particles degrade DNA and other molecules they encounter. Skin is thick enough to block alpha radiation, but Mollie and her coworkers were told to use their mouths to shape their paintbrushes.
Radium is an alkaline earth metal, just like calcium. Because elements in the same column undergo similar reactivity, the radium in the paint was shuttled to Mollie’s bones as if it were calcium. There, the radium decayed and emitted alpha particles directly to her bones and internal organs.
FYI: Beta and gamma radiation are also dangerous; they both chip away at DNA and damage living tissue. However, beta particles are smaller, move faster, and have less charge than alpha particles—they are less damaging than alpha particles by a factor of 1000. Gamma radiation is pure energy and has no mass or charge. It, therefore, has even less ionizing power than alpha or beta particles.
So, although beta and gamma radiation can penetrate skin, your body can repair damage done by small amounts of beta and gamma radiation. In fact, small amounts of gamma radiation are used to kill bacteria on food to maintain freshness and flavor without damaging the food itself.
From lab to watches to court
Not surprisingly, a number of inventors (and scientists) succumbed to cancer and other effects of radiation. The inventor of Undark, Sabin A. von Sochocky, and Marie Curie both died of aplastic anemia, in 1928 and 1934 respectively, and William J.A. Bailey—purveyor of a radium-laced health tonic—died of bladder cancer in 1949. The cause of Becquerel’s death in 1908 is unknown, but he did have severe burns on his body.
By the time USRC and the Radium Dial Company, another producer of glow-in-the-dark goods, were founded, the dangers of radioactivity were known. Management and scientists took precautions, yet the companies refused to warn Mollie and her coworkers of the hazards and encouraged the dangerous practice of pointing their brushes with their mouths because it improved productivity.
In the 1920s, some of the Radium Girls sued their employers, bringing the dangers of radiation into the public eye and establishing the right of workers to sue employers for damages from unsafe working conditions. Some of the workers received compensation, and their plight influenced the formation of the U.S. Occupational Safety and Health Administration (OSHA), which continues to define and enforce safe working conditions today.
Radiation vs. radiation
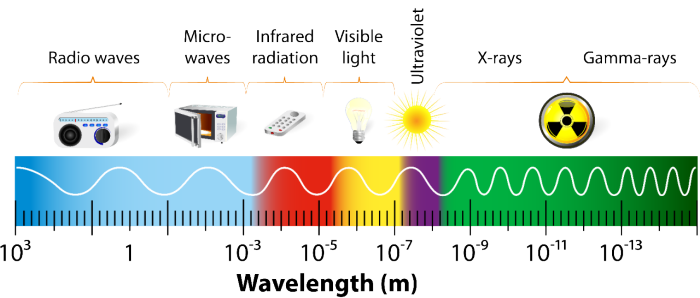
The term “radiation” refers to any energy emitted along any part of the electromagnetic spectrum, from radio waves to visible light to gamma rays. However, it is sometimes used specifically to mean the ionizing radiation resulting from nuclear decay.
Additionally, X-rays and gamma rays are also often used interchangeably. When these types of radiation were first discovered, gamma rays were defined as being higher in energy. Over time, researchers found that the energy sources used for each could be tuned to produce the same wavelengths, so X-rays and gamma rays began to cover the same energy range. Now, X-rays refer to the energy emitted when a highly excited electron relaxes, while gamma rays are produced by radioactive decay.
The Electromagnetic Spectrum
Radiation in the modern world
While history has shown us the destructive nature of radioactivity, the same properties that make radioactive materials dangerous also make them useful when proper precautions are observed. Our society’s lifesaving medical practices and devices would not exist without it. Radiation allows doctors to locate and destroy tumors, or slow their growth. When radiation is directed at cancer cells, it harms DNA, so cells stop dividing. Because radiation has the same effect on healthy tissues, doctors are strategic about delivering the radiation. For example, thyroid cancer is treated with 131I, because the thyroid selectively absorbs almost all iodine in the bloodstream.
Similarly, radioactive atoms can be attached to certain antibodies that attack specific targets on cancer cells. Tumors may also have radioactive “seeds” physically placed into or next to them or targeted with an external radiation beam.
Radiation may be used in small doses for diagnostic purposes. X-rays and CAT scans are common examples, although radioactive materials are rarely used to produce them anymore. Patients may also be injected with radioactive tracers to identify where blood is flowing or where tumors are located. Engineers use an analogous process to find leaks in metallic castings and welds or to check the flow of oil through an engine.
Radiation is also used to create electrical energy that powers nuclear reactor plants all over the world. These plants use uranium in fission reactions to produce heat and steam. The steam is then used to turn a turbine, thus creating electricity.
In consumer goods, radiation sterilizes food to remove bacteria and organisms that cause disease, clothing is treated with wrinkle-resisters, and cookware is treated with nonstick coatings. Radiation helps the treatments stick to clothing and cookware surfaces but does not make the products themselves radioactive.
In most cases, modern radioactive materials are handled exclusively by trained professionals. But you can still find radioactive species in household products. Smoke detectors contain a tiny sample of 241Am, which emits a steady stream of alpha particles. When the stream is disrupted by smoke, a sensor triggers the alarm. Don’t worry, the plastic casing is more than sufficient to contain the alpha particles. Although radium is no longer used for glow-in-the-dark watches, 3H and 147Pr are. Both are low-energy beta emitters, so the watch’s case is sufficient protection.
It’s safe to say scientists and industry have come a long way with the uses of radioactive species since the tragedy of the Radium Girls. Today, the hazards of radiation are well-understood, so we can take full advantage of its benefits.
Know your ionizing radiation
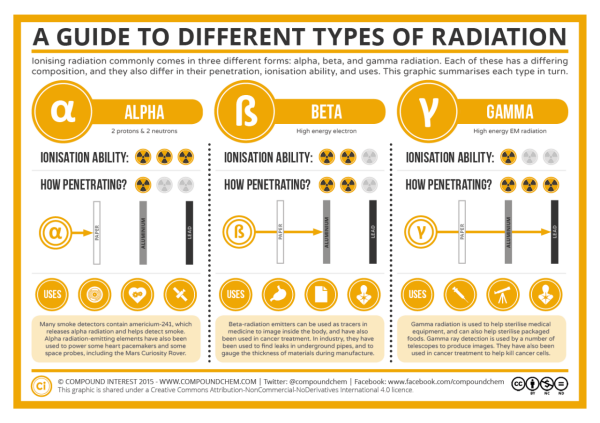
Radioactive nuclei can undergo a series of decay types, releasing radiation until a stable species results.
Alpha particle (α): two protons and two neutrons (a helium nucleus) are released from the nucleus
- Reduces the species’ atomic number by 2 and atomic mass by 4
- Penetration: minimal, can be stopped by paper, skin, clothing
- Ionization ability: high
- Examples: 241Am, 201Po
241Am → 42α + 237Np
- Uses: smoke detectors, pacemakers
Beta particle (β): electron released from the nucleus
- β decay: A neutron emits an electron, becoming a proton in the process
10n → 0-1β + 11p+
- Increases the species’ atomic number by 1, no change in atomic mass
- Penetration: moderate, can be stopped by a thin sheet of metal
- Ionization ability: moderate
- Examples: 3H, 147Pr
3H → 0-1β + 3He
- Uses: medical diagnosis, imaging of underground pipes, glow-in-the dark devices
Gamma (γ)-ray: Nucleus emits electromagnetic energy to relax from a high-energy state, accompanies other types of radiation
- Penetration: high, can be stopped by a few centimeters of lead
- Ionization ability: low
- Examples: 137Cs, 60Co
- Uses: cancer treatments, medical diagnosis, sterilization of food and equipment
Types of radiation.
Credit: Compound Interest
REFERENCES
L’Annunziata, M. F. Radioactivity: Introduction and History, From the Quantum to Quarks (2nd ed.); Elsevier: Amsterdam, 2016.
Moore, K. The Radium Girls: The Dark Story of America’s Shining Women; Sourcebooks: Naperville, IL, 2017.
Sartor, O.; Sharma, D. Radium and other alpha emitters in prostate cancer. Translational Andrology and Urology, 2018,[bf for year] 7(3), 436–444. https://doi.org/10.21037/tau.2018.02.07
World Nuclear Association. Radioisotopes in Medicine, 2019. Retrieved June 9, 2019, from https://www.world-nuclear.org/information-library/non-power-nuclear-applications/radioisotopes-research/radioisotopes-in-medicine.aspx
Contributing author: Julianna Poole