What molecule am I?
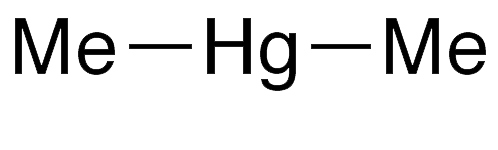
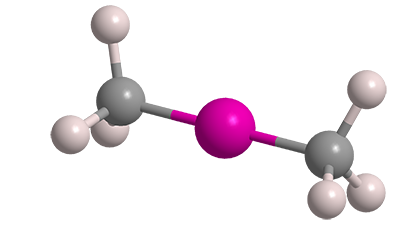
Dimethylmercury (Me2Hg) is one of the nastiest chemicals made by humans or found in nature. It was known as long ago as 1858, when English chemist/entomologist George B. Buckton isolated it during experiments with methyl- and ethylmercury compounds. It reappeared in the literature in 1899, when prominent French chemist Marcellin Berthelot found that under electrical discharge Me2Hg absorbs nitrogen gas.
In an early discovery of Me2Hg in the environment, Swedish researchers S. Jensen and A. Jernelöv reported in 1969 that it and its cationic degradation product, methylmercury1 (MeHg+), are produced in bottom sediments and rotten fish in mercury-contaminated waters. It didn’t help matters that MeHg+ had been used as a pesticide in Sweden until 1966 and contributed to the pollution.
Knowledge of the distribution of Me2Hg in oceans has been limited because of a lack of measurement methods. In a 2022 effort to combat this problem, Robert P. Mason and collaborators at the University of Connecticut (Groton) and the University of California, Santa Cruz, developed an automatic analyzer for the high-resolution measurement of Me2Hg and other volatile mercury molecules in surface ocean waters.
In 1971, two other Swedish scientists, Leif Bertilsson and Halina Y. Neujahr* at the University of Stockholm, demonstrated another way that methylmercury compounds are formed in nature. They showed that methylcobalamin2, a form of vitamin B12, reacts with mercury(II) ion in human and animal bodies to produce the deadly toxins.
The tragic death in 1997 of rising chemistry star Karen Wetterhahn at Dartmouth College (Hanover, NH) drove home the lethality of Me2Hg. While studying heavy-metal toxicity, Wetterhahn got a small amount of Me2Hg on her rubber gloves, which were later found to be permeable to the compound. She began to experience toxicity symptoms, and, less than a year after the exposure, she died. In 2022, the 25th anniversary of her death, Sam Lemonick wrote an account of Wetterhahn’s life and contributions to chemistry.
At one time, Me2Hg was used as a methylating agent in organic synthesis; but because of its toxicity, it has been replaced by safer reagents such as dimethylzinc, trimethylaluminum, and methylmagnesium halides (Grignard reagents).
For much more on the chemistry, toxicity, and environmental effects of Me2Hg, see the ScienceDirect information page.
1. CAS Reg. No. 22967-92-6.
2. CAS Reg. No. 13422-55-4.
Dimethylmercury hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Flammable liquids, category 2 | H225—Highly flammable liquid and vapor | |
| Acute toxicity, oral, category 2 | H300—Fatal if swallowed | |
| Acute toxicity, dermal, category 1 | H310—Fatal in contact with skin | |
| Acute toxicity, inhalation, category 2 | H330—Fatal if inhaled | |
| Carcinogenicity, category 2 | H351—Suspected of causing cancer | |
| Specific target organ toxicity, repeated exposure, category 2 | H373—May cause damage to organs through prolonged or repeated exposure | |
| Short-term (acute) aquatic hazard, category 1 | H400—Very toxic to aquatic life | |
| Long-term (chronic) aquatic hazard, category 1 | H410—Very toxic to aquatic life with long-lasting effects | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
Dimethylmercury fast facts
| CAS Reg. No. | 593-74-8 |
| SciFinder nomenclature | Mercury, dimethyl- |
| Empirical formula | C2H6Hg |
| Molar mass | 230.66 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 93–94 °C |
| Water solubility | Insoluble |
Molecules from the journals
The taepeenins constitute a series of 12 mostly three- and four-ring aromatic diterpenes isolated from medicinal plants belonging primarily to the genus Caesalpinia. Taepeenin F1, isolated in 2005 from C. crista, is of particular interest because of its anti-inflammatory properties. In October 2022, Fernando J. Reyes-Zurita, Rachid Chahboun, and co-workers at the University of Granada (Spain) reported an improved semisynthesis of taepeenin F starting from commercially available abietic acid2.
Tin(IV) oxide3 (SnO2), which forms in the mineral cassiterite, is the primary source for producing metallic tin and tin compounds. SnO2 has many uses, including glass and metal polishing; glass, pottery, and putty manufacture; and fabric dyeing and printing. In September 2022, Haining Tian and colleagues at Uppsala University (Sweden) described a new use for SnO2: as an electron-transport material deposited as an atomic layer in dye-sensitized nickel(II) oxide4 (NiO) films for making solid-state p-type solar cells.
Fluoxetine5, better known by its tradename Prozac, is a venerable antidepressant that has been in use since 1986. It is a selective serotonin reuptake inhibitor that is also used to combat obsessive–compulsive disorder, bulimia nervosa, and other psychiatric conditions. Like many widely used substances, fluoxetine has found its way into the environment. Last October, Guangxu Liu, Wei Shi, and co-workers at Zhejiang University (Hangzhou, China) reported that, at environmentally realistic concentrations, the drug hampers goldfish olfaction by interfering with the initiation, transmission, and processing of olfactory signals.
1. CAS Reg. No. 865314-62-1.
2. CAS Reg. No. 514-10-3.
3. CAS Reg. No. 18282-10-5.
4. CAS Reg. No. 1313-99-1.
5. CAS Reg. No. 54910-89-3.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

