Periodic Table Elements 1-20
Accompanying lesson plan:
Lesson 4.2: The Periodic Table
Note: This is an interactive periodic table. Click on an element to learn about some of its properties and how it's important in everyday life.
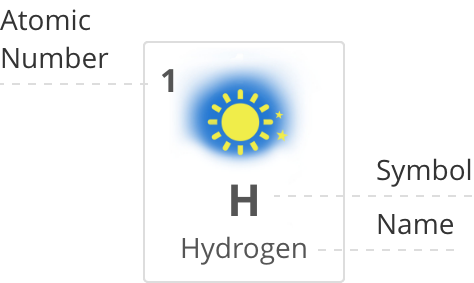
1

H
Hydrogen
Learn About Hydrogen!
Learn about hydrogen by watching the video below.
Hydrogen makes a great rocket fuel

Many of the largest rockets to lift off from Earth are powered by liquid hydrogen as the fuel. The beauty of hydrogen is that it is the lightest of all fuels and burns with extreme intensity with oxygen. The rocket shown here is the Delta Heavy Lift Rocket. The giant cylinders to the right and left of the main center rocket are filled with extremely cold liquid hydrogen and liquid oxygen.
When the hydrogen is burned with liquid oxygen, the temperature of the reaction reaches over 3,038 °C (5,500 °F). Hydrogen is not only lightweight, but also among the best fuels in providing the greatest amount of lift for the amount of fuel burned.
Hydrogen in Fuel Cells

Scientists and engineers have been working on a type of battery for cars, busses, and trucks called a hydrogen fuel cell. The fuel cell uses a tank of compressed hydrogen gas (H2) and oxygen from the air to produce electricity to run the vehicle’s electric motor. The exhaust produced from a hydrogen fuel cell is just plain water.
The advantage of a fuel cell car over a more common electric car is that a fuel cell car can be refueled quickly with hydrogen rather than taking the long time necessary to recharge an electric battery. But there are not currently many places to fill up with hydrogen and it’s a lot more expensive than the cost of recharging the battery in a standard electric car.
Another big problem with hydrogen as a fuel is the energy required to get the hydrogen in the first place. Hydrogen is attached to oxygen in water, and to carbon in petroleum and natural gas. It takes energy to separate the hydrogen from these other atoms. The energy usually comes from burning fossil fuels. So even though the exhaust from a hydrogen car is water, some environmentally unfriendly exhaust was produced to get the hydrogen to begin with.
There is still a lot of work to be done to make hydrogen fuel cell cars the low emission cars of the future.
Hydrogen peroxide

You may have seen a brown plastic bottle of hydrogen peroxide in your home. Most hydrogen peroxide used at home has a concentration of 3% which means that there is 97% water and 3% hydrogen peroxide.
A hydrogen peroxide molecule is made up of two hydrogen atoms and two oxygen atoms. It has a molecular formula of H2O2.
Hydrogen peroxide is stored in brown bottles because light can cause the molecules to react and break down into water and oxygen gas.
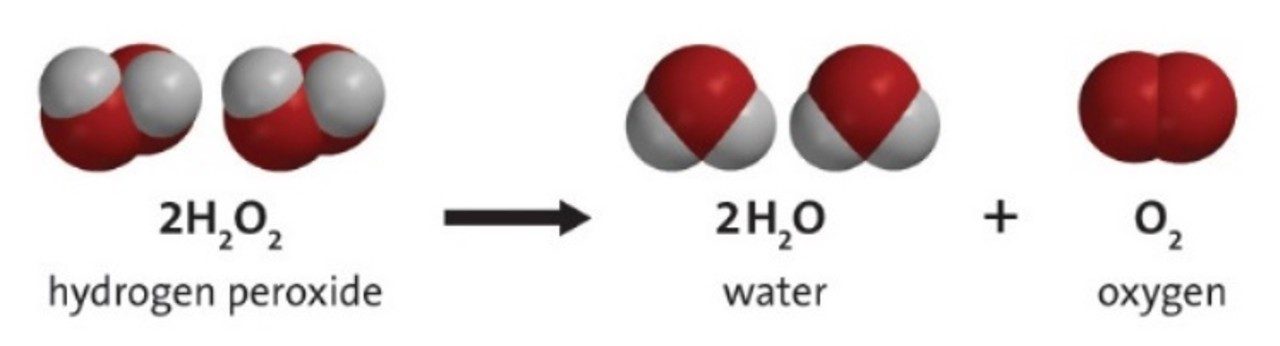
It may be surprising, but hydrogen peroxide is produced in the cells of many organisms as a by-product of certain chemical reactions. Since it can become poisonous, enzymes in the cells break it down as soon as it’s produced.
Here is a video showing the rapid release of oxygen and water from hydrogen peroxide when a catalyst is added.
After learning About Hydrogen
Try the element challenges below!
2

He
Helium
Learn About Helium!
Learn about helium by watching the video below.
Where does helium come from?
Most of the elements on Earth originated billions of years ago from the Big Bang or later from exploding stars called supernova. But helium is different.
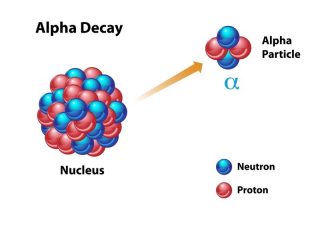
The helium found on Earth is produced from a process called radioactive decay. Radioactive decay happens when heavy atoms like uranium or plutonium release particles from their nucleus making them a different lighter atom.
Deep underground, uranium and plutonium decay by releasing particles made up of two protons and two neutrons called alpha particles. Two protons and two neutrons is the same as a nucleus of a helium atom! These helium nuclei pick up electrons and become helium atoms. The helium atoms produced in this way get trapped underground in pockets of natural gas. The gas is extracted and purified and the helium is collected and stored.
But the supply of helium can be a problem. Helium is extremely lightweight and doesn’t bond chemically to other substances. So, once helium reaches the surface of the earth, it can easily float up into the air and eventually escape the Earth's gravitational pull. It's the one element out of the entire periodic table that can just leave the planet on its own.
Helium in a Particle Accelerator
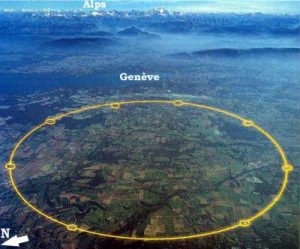
Liquid helium is a key ingredient in the largest piece of scientific equipment in the world, called the Large Hadron Collider. The collider is a giant circular tunnel almost 17 miles around, on the border between France and Switzerland.
The tunnel houses the world’s most powerful particle accelerator. It uses powerful superconducting electromagnets, cooled in liquid helium, to accelerate protons to almost the speed of light.

Liquid helium is a key ingredient in the largest piece of scientific equipment in the world, called the Large Hadron Collider. The collider is a giant circular tunnel almost 17 miles around, on the border between France and Switzerland.
The tunnel houses the world’s most powerful particle accelerator. It uses powerful superconducting electromagnets, cooled in liquid helium, to accelerate protons to almost the speed of light.
Helium in a Scuba Tank

The air we breathe in our normal everyday life is about 78% nitrogen, 21% oxygen and about 1% other gasses including argon, water vapor, carbon dioxide and a few others.
But for scuba divers who need to go deep under water, the gasses in their scuba tank need to be different. When diving deep underwater, the body is under a lot of pressure. At higher pressures, the blood absorbs more nitrogen and oxygen than it does at lower pressures of shallow dives and on dry land.
The biggest danger under these conditions is absorbing too much nitrogen which can be poisonous at high levels. For deep sea scuba diving, helium gas is mixed with nitrogen and oxygen to lower the concentration of nitrogen that could enter the blood stream. For very deep dives, the scuba tank might not contain any nitrogen at all but have 78% helium mixed with 22% oxygen.
After learning About Helium
Try the element challenges below!
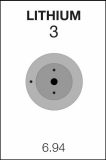
3

Li
Lithium
Learn About Lithium!
Learn about lithium by watching the video below.
Lithium in Fireworks

Most of the substances used to create colors in fireworks contain metal atoms, such as lithium, barium, copper, and sodium.
When atoms or molecules containing these metals get hot enough, they produce a particular color of light. Different substances emit different colors. Reds are produced by compounds containing the metals strontium or lithium. Barium emits green, copper produces blue, and sodium emits yellow and orange.
Lithium in Dentistry
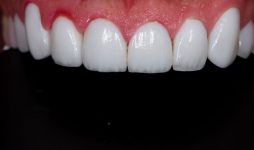
Another substance made from lithium, silicon, and oxygen is used for special applications in dentistry. The material, called lithium disilicate is used for fillings and to repair broken or cracked teeth. It is much stronger than the material that was used previously and is better able to match the color of the surrounding teeth.
Lithium in Mental Health
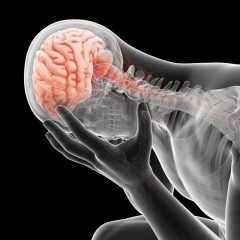
Lithium is used in medications that interact with the brain to help in treating certain serious mental health conditions. Many studies have shown that lithium can help stabilize the mood of people who suffer from a condition called bipolar disorder. Scientists are not sure exactly how lithium works but they believe that it has an effect on how the body’s nerve cells within the brain and body send and receive messages.
After learning About Lithium
Try the element challenges below!

4
Be
Beryllium
Learn About Beryllium!
Learn about beryllium by watching the video below.
Beryllium in Medicine
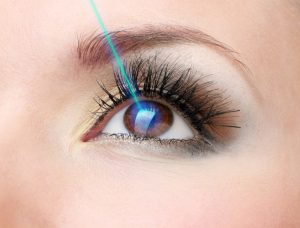
Beryllium combined with oxygen makes a compound called beryllium oxide (BO) which is very good at absorbing heat. This makes beryllium oxide useful in preventing certain types of equipment from overheating and malfuntioning. This is especially useful in very sensitive lasers used for eye and other surgeries.
When a laser is used, only a part of the energy becomes the laser beam. The rest is converted to heat. If the laser heats up too much, the laser beam might not be strong enough or could emit the wrong type of light. Beryllium oxide is the best material known for absorbing this extra heat. So beryllium oxide is used to mount and surround laser parts so that they do not overheat and can operate properly.
Beryllium’s Health Issues
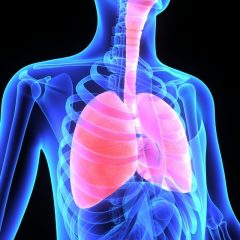
One issue with beryllium is that it is unhealthy to breathe the dust from beryllium or to have the dust contact the skin. This is a problem when workers cut, grind, or sand beryllium for a particular use. The factory should do everything it can to keep beryllium dust below a certain level and have workers wear protective masks and clothing.
The most common health effects from breathing beryllium dust are lung problems which can range from shortness of breath all the way to lung cancer. Since this problem was discovered in the 1970s, workplaces have put many regulations in place to protect workers from the hazards of beryllium dust.
Beryllium in Space

Beryllium is combined with aluminum to make a metal alloy that is very strong and lightweight. Beryllium-Aluminum holds its shape under extreme stresses in temperature, vibration, atmospheric pressure, and exposure to harsh conditions.
The unique properties of beryllium-aluminum made it the perfect material for different parts of two space rovers sent to explore the surface of Mars back in 2003. The rovers were called Spirit and Opportunity.
The rovers were designed to explore different areas of Mars for about three months. Instead, Spirit lasted seven years and Opportunity lasted fifteen years! Each rover collected images and data that helped scientists understand the history of Mars and whether life in some form may ever have exited there.
After learning About Beryllium
Try the element challenges below!
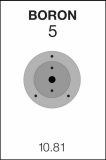
5
B
Boron
Learn About Boron!
Learn about boron by watching the video below.
Boron in Slime

Boron is used to make a substance that can stretch, bounce, flow and feel really weird – slime! Slime is usually made from a laundry powder called Borax dissolved in water, and a solution of Elmer’s glue in water.
Borax is made from a substance called sodium borate which contains boron. When sodium borate is dissolved in water, ions containing boron are formed. An ion has one or more positive or negative charges. The ion containing boron, called the borate ion, has three negative charges which come in handy for making slime.
The Elmer’s glue contains long molecules called polymers which can flow past each other in the glue. When the borax solution is added to the glue solution, the negative parts of the borate ions link to the positive parts of the glue’s polymer molecules. This prevents the molecules from flowing past each other as easily. When enough polymer molecules get hooked together in the right way, the glue solution changes from being very liquidy to a rubbery kind of stuff that we call slime!
Boron in Strong Magnets
There is a very powerful type of magnet with a difficult name to pronounce. It is called a neodymium (NEE-O-Dim-EE-UM) magnet. Neodymium is an element in the periodic table (Atomic Number 60). These super-strong magnets are an alloy made from Neodymium (Nd), Iron (Fe) and Boron (B).
The magnets are so strong that they can lift about 1,000 times their own weight. These magnets are used in cell phones, computers, electric cars, microphones, and more.
Boron in Reducing Fires

Boron has an important role in reducing property damage and injuries from fires. A compound containing boron and oxygen, called boric acid, can help keep certain materials from burning and smoldering. Boric acid is not used on clothes but is added to the cotton material in mattresses, and to certain types of home insulation and wood.
Another compound made from boron and zinc is used to make plastics less flammable. This compound is used in the plastic insulation for electrical wires, plastic car parts, and in carpeting made from synthetic materials.
Both boron compounds make it harder for materials to smolder and to burn out of control which makes our homes and lives safer.
After learning About Boron
Try the element challenges below!
6

C
Carbon
Learn About Carbon!
Learn about carbon by watching the video below.
Diamonds in Tools

Diamond is composed of carbon atoms arranged and bonded to each other in a way that gives diamond its incredible strength. When we think of diamonds, of course we think of jewelry, but diamond has another very important use. Special saw blades, drill bits, and grinding wheels have very small pieces of diamond embedded in the metal or other material they are made from.
If you look closely at the picture, you can see little bumps on the surface of the saw blade. These are the diamond chips which allow the blade to cut material like stone, cement, or metal a lot better than even the sharpest metal blade.
Carbon Nanotubes
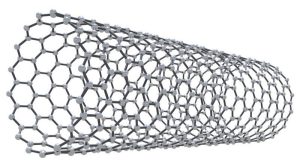
Carbon is used to create extremely tiny tubes called nanotubes. A nanotube is composed of a layer of graphite that is just one atom thick. This ultra-thin sheet of graphite, called graphine, is produced in the shape of a tube with a diameter about 1/10,000th the thickness of a human hair.
Nanotubes have many amazing properties but the one that may have the biggest impact is their use in computer technology. Nanotubes may begin to replace the silicon chips that contain the tiny transistors that transmit electric signals in the computer. Carbon nanotubes can fit way more transistors than a silicon chip of the same size.
More transistors equals more and faster processing power with less heat produced. Smaller devices, more memory, faster speeds, less overheating, and longer battery life are all big advances that nanotubes can offer.
Activated Carbon Filters

A form of carbon is used to make very effective filters for air, water, and other substances. You may have heard of “activated” carbon or activated charcoal filters. These filters contain carbon that has been treated with heat, chemicals, or a combination to give the charcoal a huge number of extremely tiny pores or spaces. All these interconnecting spaces gives each particle of activated carbon an enormous number of surfaces.

When certain types of molecules from the air or water contact the carbon, the molecules stick to these surfaces and are trapped. Substances can be added to the activated carbon so that it can be used to remove impurities from different liquids such as wine, blood, or liquids in the stomach if a poison has been ingested. It can also be used like a sponge to store different gases and to remove pollutants that come out of factory smoke stacks.
After learning About Carbon
Try the element challenges below!

7

N
Nitrogen
Learn About Nitrogen!
Learn about nitrogen by watching the video below.
Explosives

Gun powder, nitroglycerine, and dynamite all have something in common – they all explode, and they all contain nitrogen.
Explosions of substances containing nitrogen result from chemical reactions. These reactions release large amounts of energy very quickly along with gases produced during the reaction.
One of the gases produced is nitrogen (N2). In the substances in an explosive, nitrogen atoms are bonded to other atoms, but not to other nitrogen atoms. When the explosion takes place, nitrogen atoms bond to other nitrogen atoms, forming N2. This process releases a huge amount of chemical energy in the form of heat, light, sound, and expanding gases.
Car Airbags

Nitrogen is a key ingredient in the substance used to inflate a car’s air bag during an accident. The bag fills in about 3 hundredths of a second. That’s about 1/3 the time it takes for you to blink your eye!
This extremely rapid inflation is the result of an explosive chemical reaction using two compounds containing nitrogen. They are sodium azide (NaN3) which has one sodium atom (Na) and three nitrogen atoms (N). The other is potassium nitrate (KNO3) which has one potassium atom (K), one nitrogen atom (N), and three oxygen atoms (O).
When these substances are ignited by a spark from the airbag control system, a chemical reaction occurs releasing a burst of nitrogen gas which instantly inflates the airbag. So, with some chemistry and clever engineering, lives can be saved quicker than the blink of an eye.
Caffeine

Nitrogen atoms are in many important biological molecules. They are also in the famous molecule, caffeine. Most people know that caffeine is in coffee, tea, and some sodas. But caffeine isn’t just for people to drink, it actually serves an important purpose in the plants in which it’s found.
Caffeine is usually found in the highest concentrations in the seeds and leaves of coffee and tea plants, but it’s also found in some South American holly plants and in smaller amounts in many other plants. Scientists believe that the caffein acts as a natural pesticide to prevent insects from feeding on the plant.
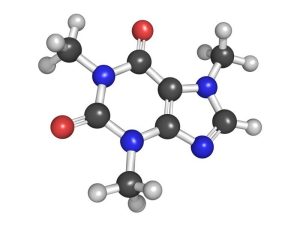
In fact, high levels of caffein are found in young coffee plants when they are most vulnerable to attack by insects. Caffeine is also found in the soil around young developing coffee seedlings. Scientists believe that the caffein may help prevent other coffee plants from growing nearby which gives the established plants a better chance to grow and develop with less competition. So caffein may be useful for people, but it turns out that it’s also good for the plants that produce it.
After learning About Nitrogen
Try the element challenges below!

8

O
Oxygen
Learn About Oxygen!
Learn about oxygen by watching the video below.
Burning hotter

If you need a fuel to burn at a very high temperature, the normal concentration of oxygen in the air might not be high enough to reach the target temperature. For the intense heat needed to cut through metal or melt metals together in welding, a source of pure oxygen is added to the fuel.
In this case, the fuel is a gas called acetylene. In the photo, notice the two hoses attached to the cutting tool, called an acetylene torch. One hose is for the acetylene and the other is for oxygen gas. The two gases work together to burn with the extreme focused heat of over 3,000 °C or over 5,700 °F!
Two sides to Ozone
The oxygen molecules we breathe from the air are made up of two atoms of oxygen bonded together (O2). There is another molecule in the air also made up of oxygen atoms. It is composed of three oxygen atoms bonded together (O3) and called ozone.
A certain amount of ozone is essential in the upper atmosphere (good ozone), but ozone at ground level (bad ozone) is a health hazard.

The ozone in the upper atmosphere, or ozone layer, absorbs a portion of the sun’s ultraviolet rays. This helps protect living organisms on Earth from ultraviolet light’s damaging effects. In the late 1970s it was discovered that substances used in refrigerators and spray can propellants contained chemicals that were causing a dangerous reduction in the ozone layer. These chemicals were banned in the late 1980s. Evidence suggests that the ozone layer has recovered and is currently at its highest level since the chemical ban was put in place.

The ozone at ground level is produced when sunlight causes pollutants from cars, trucks, and powerplants to react chemically. The resulting ozone can be a problem for people with asthma, children, older adults and people who work outside for long periods of time.
Using more electric and hybrid vehicles and the use of more renewable energy to produce electricity will help reduce the amount of ground level ozone.
Making Oxygen on Mars

Some day, The National Aeronautics and Space Administration (NASA) plans to send astronauts to land on and explore the planet Mars. But the atmosphere on Mars is about 96% carbon dioxide (CO2) and less than 1% oxygen (O2). While on Mars, the astronauts will need oxygen to breathe but they will also need a huge supply of oxygen as fuel for the trip back to Earth.
NASA has developed and tested a device on Mars that can convert the carbon dioxide in the Martian atmosphere into oxygen. The device is called the Mars Oxygen In-situ Resource Utilization Experiment, or MOXIE for short.
Since carbon dioxide has two oxygen atoms bonded to one carbon atom (CO2), the device uses a chemical reaction to get the oxygen atoms off the carbon atom to make oxygen gas (O2).
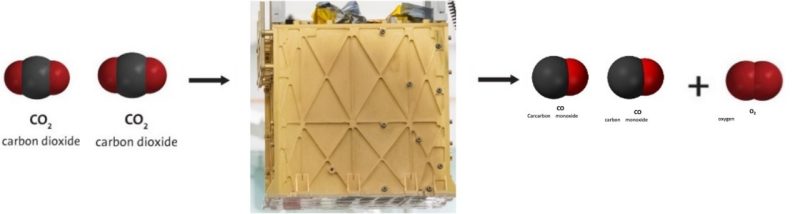
The small device has shown that it works on Mars but much larger ones will need to be developed to make enough oxygen to blast off from Mars for the return trip to Earth.
After learning About Oxygen
Try the element challenges below!

9

F
Fluorine
Learn About Fluorine!
Learn about fluorine by watching the video below.
Fluorine makes a strong acid
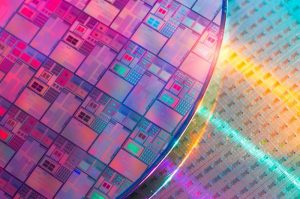
One fluorine atom and one hydrogen atom combine to make a molecule called hydrogen fluoride (HF). When hydrogen fluoride is dissolved in water, an acid is produced, called hydrofluoric acid. Hydrofluoric acid is a very strong acid that can dissolve glass. When it was first developed, scientists realized that they couldn’t store it in glass bottles, so they used containers made of rubber.
Hydrofluoric acid was used to create the first frosted lightbulbs which produce less glare than clear glass bulbs. Hydrofluoric acid is no longer used to frost lightbulbs, but it is used to clean and etch the silicon wafers that are used in the production of microchips for computers and other digital devices.
Fluorine and the ozone layer

For many years, substances made from chlorine, fluorine, and carbon called chlorofluorocarbons (CFCs) were used in refrigerators and air conditioners. They were also used as propellants in spray cans and fire extinguishers, and in the manufacture of Styrofoam.
But it was discovered that CFCs that get into the upper atmosphere react with ozone (O3) and cause a reduction in the ozone layer that protects us from the sun’s harmful ultraviolet rays. The use of CFCs for these purposes has been banned since 1996 and the hole in the ozone layer has improved a lot since that time.
Fluorine in medicine

Scientists working to develop and improve medicines have discovered different ways that fluorine can improve the medicines these new drugs. In certain cases, adding one or more fluorine atoms to a drug molecule can allow the drug to be active in the body for a longer time and give more benefit to the patient taking it. This is probably because most drugs contain carbon atoms, and the strength of the fluorine-carbon bond may prevent the medicine from being broken down too quickly in the body.
Also, adding fluorine to the molecules of some medicines allows the molecules to enter the cells of the patient more easily. This is because the fluorine-carbon bond gives the molecule the ability to dissolve in the fats of the cell membrane and enter the cell.
After learning About Fluorine
Try the element challenges below!

10

Ne
Neon
Learn About Neon!
Learn about neon by watching the video below.
How do we get neon?

Neon makes up only a very small percent (.03%) of the gases in Earth’s atmosphere. The vast majority is made up of nitrogen (78%), oxygen (21%), argon (less than 1%), and trace amounts of other gases including neon. So how do they get neon from the air to use it in Neon lights, lasers, and for other uses?
Neon and other gases are extracted from the air in a process called fractional distillation. Here’s how it works: Air is taken into a very large, tall tanks. The air is compressed and cooled down to the extremely low temperature of about -250 °C. At this temperature, each of the gases in air, including neon have condensed into a liquid. The one exception is carbon dioxide which becomes a solid at -78.5 °C.
The tank is then allowed to gradually warm. As the liquids warm, each changes from to a gas at a different temperature. When the temperature reaches -246 °C, the neon changes to a gas and can be removed and stored. Then, as the temperature increases, each gas is removed and stored: at - 196 °C nitrogen gas is removed, -186 °C argon gas is removed, -183 °C oxygen gas is removed.
So even though there is a very small percentage of neon mixed in with other gases, there is a way to extract it so its unique properties can be put to use.
Liquid neon vs. liquid helium
Materials that allow electricity to flow through them are called conductors. Copper and aluminum are good conductors and are used to make electric wires. But electricity doesn’t flow through these materials completely freely. The atoms of the material always get in the way a bit and offer some resistance to the flow of current.
But new materials have been developed called superconductors which allow electricity to flow through them with zero resistance. This is very useful in technologies that need high current to produce large magnetic fields. The only problem is that super conductors need to be made super cold. This is usually done with liquid helium.

The problem with liquid helium is that it is so light that a lot of it is needed in the cooling process. Researchers are now looking at the possibility of using liquid neon. It is more dense than liquid helium, and even though its temperature is slightly higher, less is needed to achieve the very low temperatures needed for superconductors. It is more expensive but since it can cool more efficiently than liquid helium, it is being considered for certain uses.
How Neon Lights Work
A neon light is made from a hollow glass tube filled with a small amount of neon gas that spreads out in the tube. There is an electrical contact at each end of the tube. When the neon light is plugged in and turned on, electricity passes through the tube and adds energy to the neon atoms inside. Some of the electrons in the neon atoms absorb enough energy to jump away from the nucleus of the atom. When these electrons fall back toward the nucleus, the energy they absorbed is given off as light of a certain color. For neon, the color is reddish orange.
Other lights made with gases like helium, argon, krypton, and Zenon work in the same way. These gases all give off their own characteristic color of light.
After learning About Neon
Try the element challenges below!

11

Na
Sodium
Learn About Sodium!
Learn about sodium by watching the video below.
Sodium in soap and shampoo

Sodium is a very common atom in molecules used to make soap and shampoo. One of the main ingredients in making soap is sodium hydroxide (NaOH) which is also called “lye”. Sodium hydroxide is a strong base and must be handled very carefully. When a sodium hydroxide solution is added to liquid fat or oil, a reaction called “saponification” occurs and solid soap is formed.
Sodium also appears in shampoo in a molecule called sodium lauryl sulfate. Sodium lauryl sulfate helps make the shampoo foamy and helps remove dirt and oil from hair.
The sodium part of the molecule has a positive charge while the longer part of the molecule has no charge. The uncharged end dissolves in oil and dirt while the charged end is attracted to water. When the hair is rinsed, the water pulls the oil away from the hair to clean it.
Sodium in streetlights

When heated, sodium produces a yellow flame, yellow fireworks, and the yellow color in streetlights. For many years, a light called a low-pressure sodium vapor lamp has been used in streetlights in cities, suburbs, and rural areas all over the country. They are still used in many places because they are very energy efficient and do not produce a glare at night. But they are being slowly replaced by LED lights which are even more energy efficient.
The sodium vapor lamp contains a small piece of solid sodium at one end of the bulb where there is also an electrical contact. When electricity is applied to the bulb, the sodium heats up and releases sodium atoms as a gas. The electricity excites these atoms causing them to give off their characteristic yellow light.
Salt in clean energy

Salt is being used in a very unique way to produce clean energy. In the Atacama Desert in Chile, a giant circular field of over 10,000 huge mirrors reflects sunlight to a focused area at the top of a tall tower. It’s there that 45,000 tons of salt is heated by this reflected sunlight to over 1,000 °F until it melts to become molten salt. The molten salt is stored in insulated tanks and used to heat water to make steam. This steam is pressurized and used to turn turbines that produce electricity. Unlike solar panels that produce electricity only while the sun shines, electricity can be produced at night from the very hot molten salt that was stored during the day.
It is estimated that the electricity produced can power over 300,000 homes and prevent over 600,000 tons of carbon dioxide per year from entering the atmosphere.
After learning About Sodium
Try the element challenges below!
12

Mg
Magnesium
Learn About Magnesium!
Learn about magnesium by watching the video below.
Magnesium in sparklers

Sparklers are usually made from different powdered metals like aluminum, iron, and magnesium. To burn hot enough to spark and glow, the sparkler needs more oxygen than the oxygen present in the surrounding air.
Sparkler manufacturers put an ingredient in the sparkler that produces oxygen when it burns. This extra oxygen allows the metals to burn at a temperature that makes the metals sparkle.
Magnesium and aluminum both produce white sparks, but magnesium also produces a more intense bright white light. Iron makes sparks that are more yellow or gold, and other ingredients can be added for sparks of different colors.
Magnesium fire and water

A magnesium fire is very difficult to put out even with water. The burning magnesium reacts with oxygen in the air to produce magnesium oxide. But if water is thrown on the burning magnesium, the water reacts with the hot magnesium oxide to form hydrogen gas. This is a big problem because hydrogen gas is flammable. The heat from the burning magnesium then ignites the hydrogen gas and the fire gets worse instead of being extinguished.
Firefighters are trained to use special strategies to put out the bright hot fires that can burn from parts of cars that contain magnesium.
Magnesium sulfate (Epsom salt)

Magnesium sulfate is sold in supermarkets and drugstores as “Epsom salt”. The crystals of Epsom salts are usually larger and a different shape than the cubic crystals of common table salt. Epsom salt is often dissolved in water and used as a bath for sore feet. Scientific studies have not shown that Epsom salt actually helps reduce foot pain, but people still use it as a treatment.
Magnesium sulfate is also used in the production of a type of plastic called ABS which is used for Lego bricks and for many toys and other products.
After learning About Magnesium
Try the element challenges below!
13

Al
Aluminum
Learn About Aluminum!
Learn about aluminum by watching the video below.
Aluminum used to be more expensive than silver

When aluminum was first being extracted from minerals, it was a difficult and time-consuming process. It required heating and dissolving aluminum-containing compounds and pouring the solution into a special device with electrodes at each end. Then an electric current was sent through the solution causing the dissolved aluminum to be attracted to one of two electrodes where it could be collected in small quantities. Because it was not easy to separate and purify, and such small amounts were produced, aluminum was very expensive. It was so valuable it was used as jewelry and for a while was more expensive than silver or gold.
Advances in dissolving the aluminum minerals and the ability to produce much higher electric currents allowed the process to be more efficient and the amount of aluminum produced to hugely increase until it is very inexpensive today.
Why aluminum does not rust

What we commonly call rusting is a type of corrosion. Corrosion in metals is a chemical reaction between the metal and oxygen, sometimes along with other reactants, that results in a layer of a new substance forming on the surface of the metal. Rusting happens when oxygen reacts with the metal iron. So iron, and other metals with iron in them like steel, are the only metals that can truly rust.
But other metals can also react with oxygen and corrode. In fact, aluminum is famous for not “rusting”, but it does corrode. Strangely, the thin layer of corrosion on aluminum protects it from corroding any further.
The surface of aluminum reacts with oxygen in the air to form a very hard transparent layer called aluminum oxide. So rather than falling apart because of corrosion, aluminum is protected and strengthened by it. This makes aluminum great for a lot of outdoor uses where it will be exposed to water and air.
Aluminum in antiperspirants
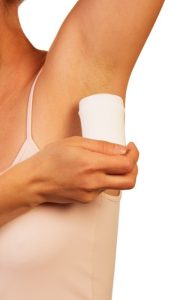
Aluminum is in a compound called aluminum chlorohydrate that is in many antiperspirants that help underarms stay dryer. The aluminum chlorohydrate temporarily plugs the pores where your sweat comes from which keeps your armpits dryer. Aluminum chlorohydrate is in antiperspirants but not in deodorants.
After learning About Aluminum
Try the element challenges below!
14

Si
Silicon
Learn About Silicon!
Learn about silicon by watching the video below.
Silicon in solar cells

Silicon crystals are used to produce microchips, but they are also used to make solar cells that convert sunlight into electricity. For the silicon to work in this way, a small amount of two different substances needs to be added to two silicon crystals. A common method is to add arsenic (which has one more electron than silicon) to one crystal and boron (which has one fewer electron than silicon) to the other. The two pieces of silicon are then sandwiched together as a solar cell. When sunlight shines on the cell, electrons move between the crystals producing an electric current.
Silicon carbide

Silicon carbide is an extremely hard substance produced from silicon and carbon. Silicon carbide is not damaged by acid and maintains strength and hardness at very high temperatures.
Silicon carbide is much harder than the hardest metal. It is used to make special tools for cutting and grinding steel and other metals and for cutting and shaping stone. Silicon carbide is also used to make automobile brake disks which need to stand up to extreme heat from friction.
Silica gel

Silica gel doesn’t really seem like a “gel”. It’s usually in the form of tiny beads or granules in a small packet in the box of a new product. The material of the packet is porous and the silica gel inside is meant to absorb water from the air in the box so that humidity does not harm the new product. Silica gel is made from silicon dioxide but processed in a way that creates a vast number of pores in each small granule. Water molecules from the air stick to the inside surface of the granules, creating a drier environment inside the box.
After learning About Silicon
Try the element challenges below!
15

P
Phosphorus
Learn About Phosphorus!
Learn about phosphorus by watching the video below.
Phosphorus in Leavening

Different substances are used to make baked foods, like breads and cakes, rise as they are baked. These substances are called “leavening agents” or just “leavening”. The most common leavening in breads is yeast. But for cakes, it’s usually baking powder.
Baking powder contains an acid and a base which react with water and the heat of the oven to produce carbon dioxide gas which causes the cake to rise. The base in baking powder is usually sodium bicarbonate (baking soda) and the acid is usually a compound that contains phosphorus.
Modern baking powder is made so that different types cause the cake to rise rapidly or more slowly. Two common phosphate compounds in baking powder are monocalcium phosphate and sodium aluminum phosphate. Both act as acids and react with baking soda to produce carbon dioxide gas to make baked foods light and fluffy.
Phosphorus in Winemaking

Phosphorus is also very useful in wine making. To make wine, yeast is added to the juice from grapes or other fruit. The yeast, which is a living fungus, consumes the sugars in the grape juice and turns some of it into alcohol. Winemaking is a complex process that requires the right temperature, type of yeast, type and amount of grape juice, amount and sugar, and other controlled conditions for the winemaking to be successful.
Wine makers use a substance called phosphoric acid as a nutrient for the yeast in their winemaking process. A phosphoric acid molecule is made up of a phosphorus atom along with oxygen and hydrogen atoms. Only a very small amount is needed to improve the health and activity of the yeast.
Phosphorus in Food

Phosphoric acid is also used in soda and jam to give them a slightly sour and tangy flavor. It also acts as a preservative to slow the growth of bacteria and mold.
Phosphoric acid is also used in making cheese from milk. The phosphoric acid interacts with protein in the milk, causing the proteins coagulate into curds. The curds are then processed further to become different types of cheese.
After learning About Phosphorus
Try the element challenges below!
16
S
Sulfur
Learn About Sulfur!
Learn about sulfur by watching the video below.
Sulfuric acid for making fertilizers

Sulfuric acid is a very important substance because it is essential for the process of making phosphate-based fertilizers for growing healthy crops. Although sulfur is not in the final fertilizer itself, the sulfuric acid is used in the beginning steps to produce a fertilizer that contains both nitrogen and phosphorous.
Sulfur for vulcanizing rubber

Sulfur is added to the rubber used to make black rubber soles for shoes, and for car and truck tires.
After its formed into shape, the tire or shoe sole is heated under pressure in a process called vulcanization where the sulfur helps the rubber become much harder and durable.
Cellophane

Sulfur is used in the production of cellophane which is the plastic wrap used to cover foods to keep them fresh. Cellophane is made mostly from the cellulose from plants, which is the material that makes up most of the plant cell wall. But to process the cellulose to produce cellophane, sulfur and sulfuric acid are used in different steps along the way.
After learning About Sulfur
Try the element challenges below!

17
Cl
Chlorine
Learn About Chlorine!
Learn about chlorine by watching the video below.
Chlorine as a cleaner

The chlorine-containing molecule used to disinfect water (sodium hypochlorite) also goes by the common name “bleach” and has many other important uses. Bleach is used in detergents to make clothes and other material whiter. Bleach is also used in hospitals, kitchens, and bathrooms to clean and kill germs on many different types of surfaces.
Chlorine in our immune system
Research has shown that chlorine bleach is produced by our body’s immune system to help fight infection. When bacteria, viruses, or other foreign organisms enter the bloodstream, white blood cells called T-cells attack them. The T-cells use bleach and other chemicals to destroy the proteins in the organism which kills them.
Chlorine in PVC

A large amount of chlorine is used to make a plastic called polyvinyl chloride or pvc. This plastic is commonly used for pipes, construction materials, and insulation for electrical wires.
Issues have been raised about the health and environmental safety of the manufacture, use, and disposal of pvc so changes in the plastic, or new materials to replace it may need to be developed.
After learning About Chlorine
Try the element challenges below!

18

Ar
Argon
Learn About Argon!
Learn about argon by watching the video below.
Argon in historic preservation

Because argon doesn’t react with other substances, it is used to preserve historic documents. If exposed to air, molecules in the paper, parchment, or ink could react with oxygen causing the document to change color and deteriorate.
That’s why the original Declaration of Independence, Bill of Rights, and Constitution of the United States are displayed at the National Archives in a case filled with argon gas.
These three documents were written on parchment which is made from animal skin. The ink was made from natural dyes from oak trees mixed with iron. None of these substances will react with argon so the ink and parchment should be preserved for many years to come.
Argon in keeping wine fresh

When wine is produced and stored in barrels and bottles, there is always a small “air space” at the top where the liquid doesn’t completely fill the container. Some wine makers inject argon gas into the bottle or barrel of wine so that the small space at the top will contain argon gas instead of regular air.
Since argon won’t react with any of the ingredients in the liquid, the wine will stay fresher for a longer time.
Argon close to home

Most of the elements on Earth and in the periodic table come from somewhere else in the universe. Some elements formed soon after the original Big Bang, others were formed in stars, and others in different phases of dying and exploding stars.
But the argon on Earth is produced right here on Earth from potassium. A small percentage of potassium is radioactive and can change from potassium to argon. There is A LOT of potassium on Earth which is why even a small percent changing into argon still produces almost all the argon on Earth.
After learning About Argon
Try the element challenges below!
19

K
Potassium
Learn About Potassium!
Learn about potassium by watching the video below.
Iodized salt

If you look for salt at the store, you usually have a choice of buying salt or “iodized” salt. “Iodized” means that the salt has been treated in some way so that it contains a small amount of iodine. This is usually accomplished by adding the compound potassium iodide (KI) to the salt.
The addition of potassium iodide is important because iodine is an essential nutrient for the body. Iodine affects the functioning of the thyroid gland which helps control how your body uses the food you eat.
“Elephant’s Toothpaste”

Potassium iodide is also used in a chemical reaction demonstration often called “Elephant’s Toothpaste”.
In the reaction, liquid dish detergent is added to hydrogen peroxide. Potassium iodide is then added to the mixture. The potassium iodide acts as a catalyst which causes the reaction to happen extremely quickly. The products of the reaction are heat and oxygen gas resulting in foam rapidly moving out of the container.
Breathing equipment

Potassium and oxygen can form a very interesting and useful substance called potassium dioxide (KO2), also called potassium superoxide. Potassium dioxide is a yellow solid that reacts with both carbon dioxide and water in the air to produce oxygen. This unique reaction is used to remove carbon dioxide and produce oxygen in devices called rebreathers.
A rebreather is used in a situation where a person must breathe in their own exhaled breath again. Certain situations on submarines, scuba dives, firefighting, or in astronaut space suits may require use of rebreathers. Since potassium dioxide reacts with the carbon dioxide and moisture in breath to produce oxygen, it is perfect for this use.
After learning About Potassium
Try the element challenges below!
20

Ca
Calcium
Learn About Calcium!
Learn about calcium by watching the video below.
Gypsum
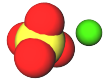
A very useful mineral containing calcium is called gypsum. Instead of calcium carbonate like limestone, gypsum is calcium sulfate, which is 1 calcium ion and 1 sulfate ion.

Huge amounts of gypsum are mined and processed to become plaster and sheet rock for building walls in homes, businesses, and other buildings.
A very unusual form of gypsum has been discovered growing as enormous crystals in a cave in Mexico.
Chalk

In addition to sheetrock and plaster, calcium sulfate (gypsum) is often used to make sidewalk chalk and chalk for blackboards.
Gypsum is a great material for molding into many different shapes. After it is crushed into a powder and dehydrated, water is added to make a thick mixture. The mixture is then poured into a mold. When the water evaporates again, the gypsum returns to its naturally hard state in the shape of the mold.
Gypsum is one of the few natural materials that can be dehydrated and rehydrated to give this result.
Calcium supplements
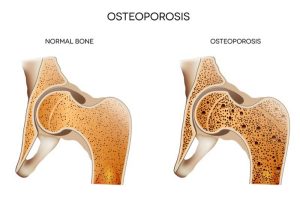
People whose bones become weak from a condition called osteoporosis are advised to exercise, get enough vitamin D, eat a healthy diet, and possibly take calcium supplements. Vitamin D helps the body absorb the calcium from food and from the supplement that’s in the bloodstream.
The two most common calcium supplements are calcium citrate and calcium carbonate. Calcium citrate is made up of calcium and citric acid which is the acid in citrus fruits like oranges and lemons. Calcium carbonate in the supplements is usually from limestone or from processed seashells.
After learning About Calcium
Try the element challenges below!