The atoms and molecules that make up all substances are connected in a particular way for each substance. In crystals, the particles repeat in a pattern that gives the crystal its special shape. Some crystals look very similar but here are a couple of ways to tell them apart.
Here's what to do:
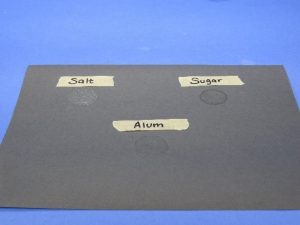
- Use masking tape and a pen to label three areas on your black paper “Salt”, “Sugar”, and “Alum”. Place a very small amount of each type of crystal on its labeled area of the black paper.
- Use a magnifier to look very closely at each type of crystal. Even though they look similar, do you notice any differences?
Don’t throw away the construction paper because you’ll need it for another activity.

What to expect
The salt may look a little grayer than the sugar which may look whiter than the salt. The alum may sparkle a little more and look a little shinier than the salt or sugar.
Try a Dissolving Test
Another way to see how similar or different the crystals are is by comparing them in a dissolving test.
What you'll need:
- Salt
- Sugar
- Alum (In spice area of the grocery store)
- Water (room temperature)
- 3 Clear plastic cups
- Measuring spoons
- Masking tape
Here's what to do:
- Use masking tape and a pen to label the three cups “Salt”, “Sugar”, and “Alum”. Place 1/2 teaspoon of sugar, salt, and alum into their labeled cups.
- Add 2 teaspoons of room temperature water to each cup. At the same time, you and your adult partner should swirl the cups for about 30 seconds.

- You and your adult partner should swirl the cups for another 30 seconds and check the results to see which substance dissolved the most and which dissolved the least.
Don’t throw away the solutions because you can use them in the next activity.
What to expect
All or almost all the sugar should dissolve. There should be some salt and some alum left undissolved in their cups. There should be more undissolved alum than undissolved salt.

Try a Construction Paper Test
If it was a little tricky to tell the difference between salt and alum in the dissolving test, give the construction paper test a try.
Be safe
Please review the safety instructions on page 1 before proceeding.
What you'll need:
- Salt, sugar, and alum solutions from the previous activity
- Black construction paper from the first activity
- 3 cotton swabs
Here's what to do:
- Place a cotton swab in each solution. On the construction paper under “Salt”, use the swab to make a round area of sugar solution about the size of a quarter.
- Re-dip the swab in the salt solution and put another layer on the same spot. Repeat this one more time so that you’ve put solution on the spot a total of three times.
- Repeat steps 1 and 2 for the sugar solution and the alum solution.

Did you notice any differences in the way the solutions looked on the paper?
What to expect
After waiting 20-30 minutes, the solutions will absorb and evaporate. The salt solution leaves a spot of tiny crystals of salt. The sugar solution leaves a dark spot and the alum solution leaves a lighter spot.
What's happening in there?
The crystals of different substances are made up of different atoms and molecules. It’s the atoms and molecules and how they are arranged that give different substances their characteristics. Since the salt, sugar, and alum are made from different atoms and molecules, they look different when their solutions absorb into and evaporate from the construction paper.
What else could you try?
You can compare salt, sugar, and alum by mixing them with dirty water and seeing if there is any difference between the way the water looks afterwards.
What you'll need:
- Labeled plastic cups
- Water
- Dirt
- Salt
- Sugar
- Alum
- ½ teaspoon
- Plastic spoon for stirring
Here's what to do:
- Add water to the three cups until they are about ¾-full.
- Add about 1 teaspoon of dirt to each cup and stir until the water looks dirty in all the cups.
- Add ½ teaspoon of salt to its labeled cup, ½ teaspoon of sugar to its cup, and ½ teaspoon of alum to its cup.
- Stir the mixture very well in all three cups.
- Let them sit undisturbed and check them in about 15 – 20 minutes.
What to expect
The water in the container with the alum should look clearer than the water with the salt or sugar.
This test shows that alum has a characteristic property of being able to make dirty water clearer. It is also another way of telling that the alum is different from salt and sugar even though it looks similar to both.