Why does soda shoot out of the can when
you open it?
When soda companies add carbon dioxide gas to a soda mixture, the water is very cold so it can hold a lot of gas. They also use pressure to put more gas in the water than it could normally hold at that temperature.
But when a soda can warms up a bit or when the can is shaken, that extra gas is really ready to come out. So when you open up the can and release the pressure, splooosh!

Why does soda bubble so much when you pour it on ice cream to make an ice cream soda?
Ice cream may look smooth to you, but if you could see it with a powerful microscope you would see something different. Throughout the ice cream there are very tiny ice crystals. The gas molecules from the soda gather on these tiny crystals and become bubbles of gas.
If the ice is already wet, some of the points are smoother so not as many bubbles form.

Soda Pop Molecules
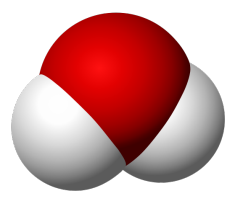
Water
A water molecule is made up of one oxygen atom and two hydrogen atoms. Water makes up about 95% of most sodas.
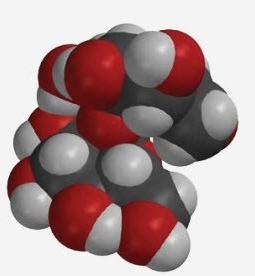
Sugar
The sweetener in most soda is a mixture of a sugar called "glucose" and another called "fructose". In fact, when these two sugars are attached to each other, it makes another sugar called "sucrose." Sucrose is the regular sugar you use in iced tea or in baking. It is made from carbon, oxygen, and hydrogen atoms.
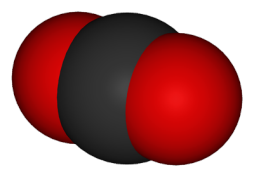
Carbon Dioxide
Carbon dioxide is made from one carbon atom and two oxygen atoms. The molecules of carbon dioxide are thoroughly mixed and dissolved into the water in the soda pop. When you open a soda can or bottle, the carbon dioxide will begin to come out of the soda and into the air. Eventually enough will come out and the soda will become flat.