Photochemistry
Are Excimers Emissive?
by Ben Zhong Tang
May 16, 2016
The answer to the question has been “no” since the early 1950s, when Theodor Förster discovered the photophysical effect of concentration-quenching of fluorescence in a concentrated pyrene solution. The light emission of rodlike and disc-shaped molecules (e.g., pyrene and perylene) is often weakened or quenched in concentrated solutions.
The concentration-quenching effect was believed to be caused by the formation of sandwich-shaped excimers (see information box) and aided by collisional interactions between the aromatic molecules in the excited and ground states. The effect is “common to most aromatic hydrocarbons and their derivatives”, as summarized by John B. Birks in his classic 1970 book, Photophysics of Aromatic Molecules. Recently, however, some research groups reported efficient luminescence from excimers of pyrene and perylene derivatives, posing a challenge to the general belief that excimers are nonemissive.
Excimers
An excimer (originally short for excited dimer) is a transient combination of two identical molecules. In the dimer, one molecule exists in an electronically excited state whereas the other is in its ground state. When the excimer relaxes back to the ground state, it dissociates, releasing heat or emitting light. An excimer typically lasts for only a few nanoseconds.
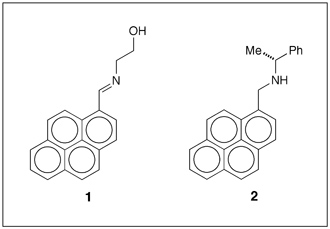
Pyrene derivatives
Fu-Hsiang Ko and co-workers at the National Chiao Tung University (Hsinchu, Taiwan) synthesized a Schiff base (1 in Figure 1) via a one-pot condensation reaction. In acetonitrile solution, the monomer of 1 emits weakly at 417 nm because of quenching by photoinduced electron transfer (PET) from the aldimine nitrogen to the pyrene ring.
But when molecules of 1 aggregate in acetonitrile–water mixtures with high water content, the emission is red-shifted to 465 nm and enhanced ≈10-fold by the formation of pyrene excimers. In addition, coordinating 1 with a trivalent metal ion (Fe3+, Cr3+, or Al3+) in a 2:1 ratio gives complexes of the form 1–M3+–1. The complexation shuts down the PET process, making the excimer complex highly fluorescent. (Sens. Actuators B DOI: 10.1016/j.snb.2016.02.136).
The fluorescence behavior of pyrene derivative 2, prepared by Junfeng Li, Wen-Yong Lai, and co-workers at Nanjing University of Posts & Telecommunications (China), is similar to that of 1. Adding water to a tetrahydrofuran (THF) solution of 2 red-shifts its light emission by forming pyrene excimers via strong π–π stacking interactions in the nanoaggregates of 2.
At 95% water content, the emission from excimers of 2 is 145-fold more intense than that of its monomer in THF solution. Moreover, aggregated 2 exhibits circularly polarized luminescence (CPL) because of its chiral molecular structure. The CPL of thin films of 2 is stronger than its monomeric solution. The dissymmetry factor of the films is about one order of magnitude greater than that of the monomer. (Tetrahedron Lett. DOI: 10.1016/j.tetlet.2016.02.015)
A perylene derivative
The perylene system has more fused aromatic rings than pyrene; and its larger disc shape makes its molecules form excimers more easily. Pawel Krukowski and colleagues at Osaka University synthesized perylene derivative 3 (Figure 2) and investigated its luminescence behavior.
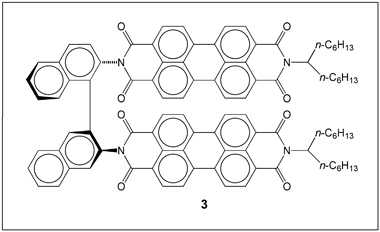
The isolated molecules of 3 in chloroform solution have emission peaks at 545.5 and 586.2 nm with a shoulder at 639.8 nm. When 3 is adsorbed onto metal surfaces, it forms molecular clusters, causing its emission to red-shift into the near-infrared spectral region because of excimer formation aided by strong dipole–dipole and π–π stacking interactions with neighboring molecules. The light emission from the excimer clusters is much stronger than that from the monomer. (J. Phys. Chem. C DOI: 10.1021/acs.jpcc.5b12313)
A mechanistic hypothesis
Conventional wisdom dictates that excimer formation leads to emission quenching. Strong light emissions from the pyrene and perylene derivatives (1–3) discussed above seem to contradict this commonly believed photophysical “law”. The questions to be answered here are: How is light emission is quenched by excimer formation in “general” systems? And why are the excimers of 1–3 more luminescent than their monomers?
“General” systems are often associated with Förster’s concentration-quenching effect. In excimers formed in concentrated solutions, the neighboring molecules in the ground and excited states are loosely held together through noncovalent intermolecular π–π interactions. Dynamic molecular motions can be activated by all sorts of processes, such as stochastic thermal agitation and Brownian motion in liquid media.
These intermolecular motions generate friction between the excimer and the medium and bring about a transformation from photonic to thermal energy. The resulting heat dissipation leads to the emission-quenching effect commonly observed in concentrated solutions.
In solid nanoaggregates suspended in aqueous mixtures, especially those that are crystalline, physical constraints and structural rigidity make it difficult for the compact excimers to undergo intermolecular and inter-ring motions. This restriction of intermolecular motion enhances excimer emission. The strong π–π stacking interactions bathochromically shift the emission color.
These scenarios indicate that whether light emission is quenched or enhanced is not determined by the excimer itself but by its packing structure. Loose excimers may not be emissive, but compact ones can be highly luminescent.

