Appearing & Disappearing Ink
Ages
8 - 12 years
Activity Time
Preparation: 10 mins
Activity: 5-20 mins (hands-on)
Group Size
* Participants work in pairs/trios
* 1 facilitator per 5 groups
ACS Student Chapter at Heidelberg University Presents: Appearing & Disappearing Ink
- Indicators: Turmeric as an acid-base indicator that changes from yellow to red in basic solution
- Acids and bases: The ammonia in window cleaner is a base
- Much of this activity is spent waiting for paper to dry. Prepare some samples in advance or have another activity for participants to do while they wait.
- Potential hazards include:
- Broken glassware
- Flammables (isopropyl alcohol)
- Inhalation (isopropyl alcohol) - Conduct your own RAMP assessment prior to presenting this activity.
Each group should be provided:
- 2 - 3 pieces of yellow or dark yellow construction paper
- ⅛ tsp (0.6 mL) powdered turmeric
- 1 tsp (5 mL) rubbing alcohol (isopropyl alcohol)
- Spray bottle of ammonia- based window cleaner
- 1 tbls (15 mL) water, plus additional for washing
- 2 - 3 cotton swabs or small paintbrushes
- Small bowl or cup
- Measuring spoons
- Any additional materials identified in your RAMP analysis
Prior to Activity
Customize Activity to Venue
- Work in a well-ventilated area.
- Review RAMP safety worksheet for this activity.
- Revise procedure to adapt to your specific venue and participants.
- List appropriate procedures for accidents, emergencies
Identify Safety Practices
- Wear appropriate personal protective equipment (e.g., goggles, gloves, etc.).
- Secure loose hair, clothing.
- Prohibit eating, drinking.
- Clean work area, wash hands after activity.
- Other practices identified in RAMP worksheet
Prepare Materials
- Collect materials.
- Identify something to do with participants while their paper dries.
On-Site
For each group:
- Arrange materials in a well- ventilated area.
- Ensure each participant has room to create their own picture or message.
-
Introduce Appearing and Disappearing Ink
Instructions
- Explain that participants will be using chemistry to write secret messages or draw hidden pictures
-
Prepare Ink
Instructions
Direct participants to:
- Mix together ⅛ tsp turmeric powder, 1 tsp rubbing alcohol, and 1 tbls water in the bowl or cup.
- Do your best to break up any clumps of turmeric.
Talking Points
- Why do you think rubbing alcohol is used in this activity?
-
Create Picture or Message
Instructions
- Dip the paintbrush or cotton swab into the turmeric solution then brush it onto the paper in your desired pattern.
- Allow the paper to dry.
Talking Points
- How does the turmeric slurry look?
- Why can’t you see what you drew?
- What do you know about acid-base indicators? (See Explore the Chemistry)
-
Reveal Picture or Message
Instructions
Direct participants to:- Spray paper with window cleaner.
- Wait for the window cleaner to dry.
Talking Points
- What happened to your drawing or message as the paper dried? Why do you think that happened?
- Why do you think yellow paper is necessary for this activity?
- Are there other papers, spices, or indicators you could use?
-
Clean Up
Instructions
- Dispose of all solids from this activity in the trash.
- Dispose of all liquids down the drain.
- Clean all work surfaces with water or a damp cloth.
- Wash hands thoroughly.
Here are some key themes to explore with the audience once they've completed the activity. Adjust the details to match the level of your audience.
Chemists classify substances as acids or bases. Lemon juice and vinegar are both examples of acids. On the other end of the spectrum are bases, like baking soda or the window cleaner you used in this experiment. Some substances are neutral, meaning they are neither an acid nor a base, like water.
Acid-base indicators can tell us whether something is an acid, a base, or neutral. Indicators change color depending on whether they are mixed with an acid or a base.
Turmeric is a powdered spice made of the crushed roots of a plant. It contains a natural indicator called curcumin that stays yellow-gold in an acid like lemon juice or in neutral solutions like water. (This form is also referred to as "diketo," because it contains two carbons double- bonded to oxygens, aka, ketone groups.)
In a basic solution, curcumin changes to a red-orange form. (One of the ketone groups is hydrated to an alcohol group, aka the "enolate" form.) Curcumin dissolves better in organic solvents than water. Adding rubbing alcohol (which is 70% isopropyl alcohol in water)helps it dissolve. It also speeds up the evaporation to help the paper dry out faster.
The window cleaner you used in this experiment contains a stinky gas called ammonia (NH3), which is a base that dissolves easily in water. When sprayed with ammonia, the turmeric powder turns red-orange, revealing the message against the yellow paper! When the ammonia evaporates into the air, the original yellow-gold color returns.
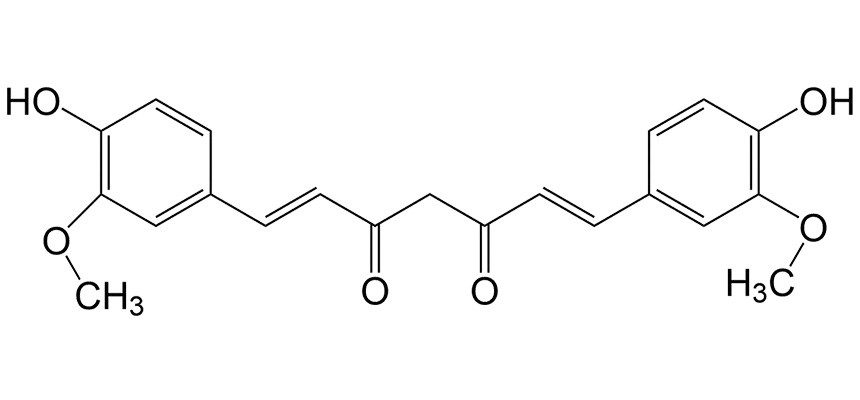
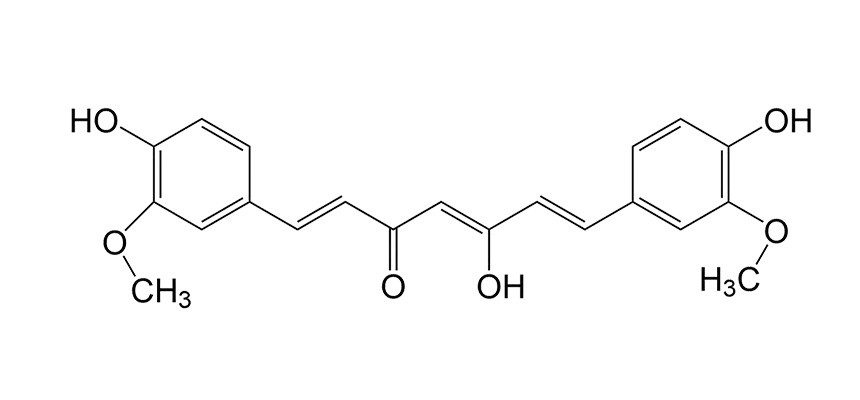
References
- American Chemical Society, 2023
- ACS Student Chapter at Heidelberg University





