Chemistry That Glows
Ages
5 - 18 years
Activity Time
Prep: 20 mins
Activity: 5-10 mins
Group Size
25-250 observers
ACS Student Chapter at the University of Pittsburgh at Johnstown Presents: Chemistry That Glows
- Chemiluminescence: Reaction of luminol with oxidants emits light
- Forensics: The chemiluminescence of luminol can be used to detect certain biological compounds, even after cleaning
- Other applications: Chemiluminescent reactions are used in glow sticks and certain plants and animals
- Present in a laboratory setting or venue in which presenters and spectators can be separated by >10 ft.
- Best viewed in a dark setting.
- All cloth used in this activity may become permanently bleached.
- Potential hazards include:
- Acids and bases
- Broken glassware
- Inhalation hazards
- Oxidizers
- Spills and splashes - Conduct your own RAMP assessment prior to presenting the activity.
- 100 mL of commercial bleach, or 5-6% NaOCl solution
- 4 g NaOH (lye)
- 0.46 g luminol (5-amino-2,3- dihydrophthalazine-1,4-dione)
- 2 L distilled, deionized water, plus extra for rinsing
- One (1 L) storage container, tinted or opaque
- One (1 L) storage container, plastic, preferably HDPE
- Two (100 mL) graduated cylinders
- 500 mL beaker or Erlenmeyer flask
- Two spray bottles
- Labels
- Cloth towel or shirt
- Additional materials you identified in your RAMP analysis
- Optional:
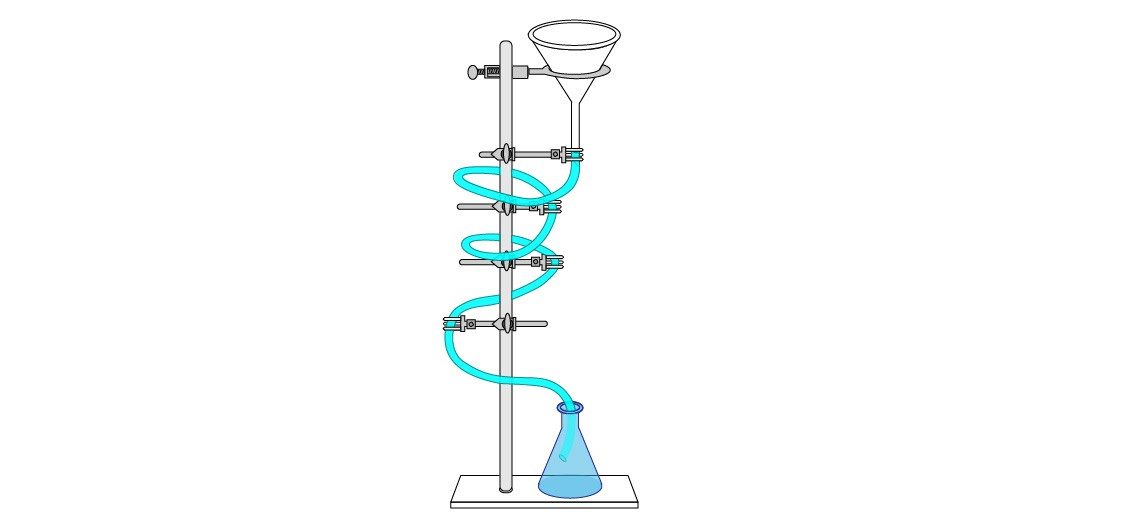
- Iron ring stand
- Iron ring
- Large funnel
- 3 ft of clear, colorless plastic tubing that fits snuggly around the funnel’s stem
- Clamps
Prior to Activity
Customize Activity to Venue
- Work in a well-ventilated area.
- Revise procedure to adapt to your specific venue and participants.
- List appropriate procedures for accidents, emergencies.
Identify Safety Practices
- Wear appropriate personal protective equipment (e.g., goggles, gloves, etc.).
- Secure loose hair, clothing.
- Prohibit eating, drinking.
- Clean work area, wash hands after activity.
- Ensure a minimum of 10 ft between presenters and audience
Prepare Materials
- Dilute 100 mL of commercial bleach (usually 5-6% NaOCl) to a 1 L solution with water. Store in a tinted or opaque bottle for up to 1 month.
- Dissolve 4 g NaOH in water and dilute to 1 L. Add 0.46 g luminol and stir until dissolved. Store in plastic bottle.
- Label one graduated cylinder and one spray bottle, “bleach solution.”
- Label one graduated cylinder and one spray bottle, “luminol solution.”
On-Site
- Prepare a space in which:
a. The lights can be dimmed
b. The audience is at least 10 ft away from the activity
c. Spills can be easily contained - Fill graduated cylinders with 100 mL each of indicated solution.
- Set out beaker/flask.
- (Optional) Position the iron ring at the top of the ring stand. Set a funnel in the ring with the stem facing down. Affix one end of tubing to the stem of the funnel. Use the clamps to secure the tubing in a loose spiral around the ring stand. Set the loose end of the tubing in the bottom of the beaker/flask.
- Fill each of two spray bottles about 1/3 full with the solution indicated on its label.
- Position towel or t-shirt so that it can be easily seen by the audience.
-
Introduce Concept of Chemiluminescence
Instructions
- Explain that some reactions emit light.
Talking Points
- Have you ever seen fireflies?
- Where else do you see examples of glowing in nature, also known as bioluminescence?
-
Demonstrate Luminol Reaction
Instructions
- Turn off lights.
- Simultaneously pour 100 mL each of bleach solution and luminol solution into the beaker/flask
- Optional: simultaneously pour the bleach and luminol solutions into the funnel so the audience can see the reaction as it moves down the tube. - Turn lights back on.
Talking Points
- What do the solutions look like before they are mixed?
- What happens when the solutions are mixed?
- Why is bleach used?
-
Demonstrate Use of Luminol in Forensics
Instructions
- Turn off lights.
- Spray towel/shirt with luminol solution.
- Spray towel or shirt with bleach solution.
Talking Points
- How might this reaction be used outside of the lab?
- Why do you think luminol might not be the first analysis used at crime scenes?
-
Clean Up
Instructions
- Dispose of all solids from this activity in the trash.
- Dispose of all liquids down the drain with lots of water.
- Clean all work surfaces with water or a damp cloth.
- Wash hands thoroughly.
Here are some key themes to explore with the audience once they've completed the activity. Adjust the details to match the level of your audience.
What makes luminol glow?
When the chemical energy of a reaction is converted to visible light energy, the resulting glow is called “chemiluminescence.” Chemiluminescent reactions are what make glow-sticks and some road safety lights glow.
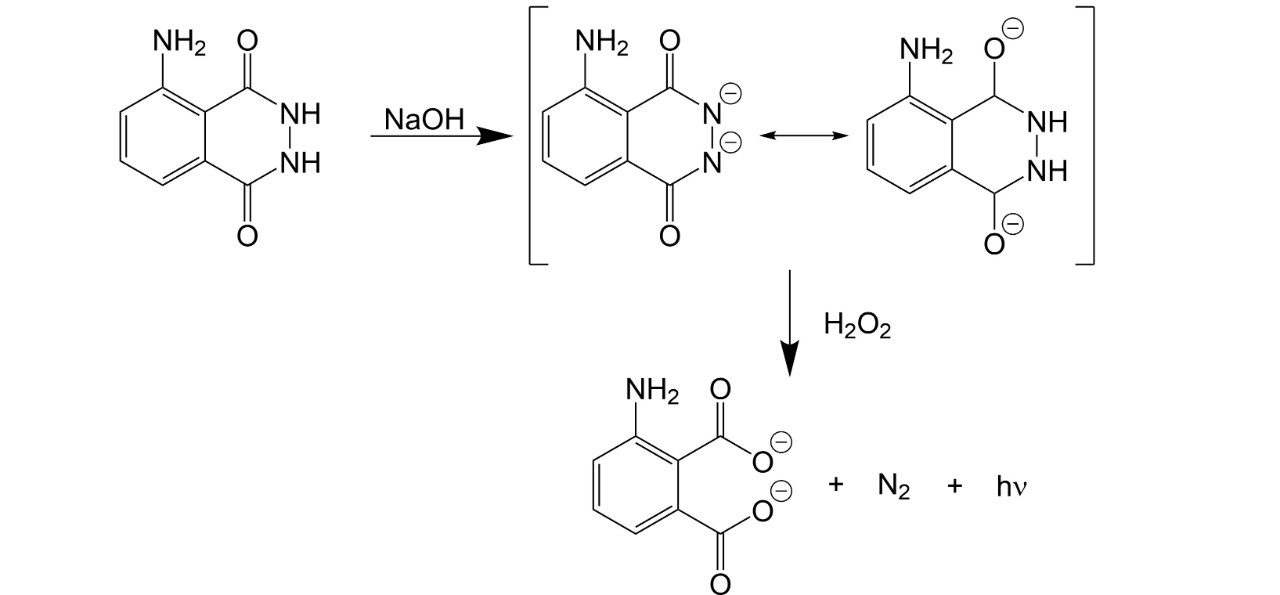
When luminol is dissolved in a base, such as NaOH, the H+ on its nitrogens are stripped off, leaving a dianion (i.e., a molecule with two negative charges). The dianion forms resonance structures, which stablizes it just long enough to be oxidized by the bleach, which removes the nitrogens to form a dicarboxylate ion and N2.
The oxidized luminol is left with a lot of energy, which it releases as light. Similarly, glow sticks rely on the oxidation of a trichlorosalicylate oxalate ester in a basic solution by hydrogen peroxide.
Luminol and forensics
Hemoglobin, an oxygen-carrying protein in blood, catalyzes the luminol oxidation reaction for a strong glow. Luminol is very sensitive and able to detect trace amounts of blood, even latent blood that has been cleaned or removed.
Luminol also reacts with other oxidizing compounds, such as those found in urine or saliva (even horseradish), so a positive luminol test is usually followed by one that is more specific for blood. Because the application of luminol can dilute any blood that may be present or damage other evidence, other non-destructive techniques are generally used.
Chemiluminescence in nature
When a chemiluminescent reaction occurs in an living organism, the phenomenon is called “bioluminescence.” Bioluminescent organisms include fireflies, some fungi, and certain jellyfish, bacteria, algae, and saltwater fish. These organisms either produce or absorb luciferin, a light-emitting compound.
References
- American Chemical Society, 2023
- ACS Student Chapter at University of Pittsburgh at Johnstown
- Procedure developed by Dr. Marsha Grimminger, University of Pittsburgh at Johnstown, based on resources from Science in Motion, Juniata College