Rainbow Tornado
Ages
12 - 18 years
Activity Time
Preparation: 10 - 15 mins
Activity: 10 - 15 mins
Group Size
* Participants work in pairs/trios
* 1 facilitator per 5 groups
ACS Student Chapter at Georgia Gwinnett College Presents: Rainbow Tornado
How many times can you add vinegar before this color-changing chemical reaction comes to an end?
Review with teacher or group leader to identify the appropriate concepts for your group:
- Acids and bases: Acids produce H+ ions in solution while bases produce OH-
- Neutralization: Acids and bases react to form neutral solutions.
- Solubility: Mg(OH)2 is slightly soluble in water but dissolves more easily with acid
- Indicators: Acid-base indicators change colors to denote changes in pH
- Equilibrium: With each addition of acid, the system shifts to restore equilibrium in accordance with Le Châtelier's Principle
If using a magnetic stir bar, be sure to clamp the flask firmly in place to avoid spills.
- Potential hazards include:
- Acids and bases
- Broken glassware
- Spills and splashes
- Conduct your own RAMP assessment prior to presenting this activity.
For 15 groups:
- 2 – 26 fl oz bottles milk of magnesia*
- 3 L water
- Universal indicator or red cabbage indicator†
* Found with the antacids in most grocery stores and drugstores.
† Make red cabbage indicator: Shred 1-2 red cabbage leaves and freeze 1 hour in a zip-close plastic bag. Add 1/3 cup warm water, reseal bag, and squish the leaves with hands. When liquid is as dark as possible, decant and discard solids.
- 1 – 1.32 gal bottle white vinegar
- 45 large clear, colorless plastic cups
- 15 long-handled spoons or glass stirring rods‡
- 15 tablespoon measuring spoons or medicinal measuring cups
‡ Magnetic stirplate and stir bars may be used; replace 15 plastic cups with Erlymeyer flasks and add clamps to secure.
- 15 copies of a pH color chart to hand out, or 1 copy to project to the class
- Any additional materials identified in your RAMP analysis
Prior to Activity
Customize Activity to Venue
- Review RAMP safety worksheet.
- Adapt procedure to your venue and participants.
- Review activity with the teacher or group leader.
- List appropriate procedures for accidents, emergencies.
Identify Safety Practices
- Wear personal protective equipment (goggles, gloves, etc.).
- Secure loose hair, clothing.
- Prohibit eating, drinking.
- Clean work area, wash hands after activity.
- Additional practices identified in RAMP worksheet.
Prepare Materials
- Collect materials
- For each group, label a set of 3 cups with: vinegar, water, and milk of magnesia.
On-Site
For each group:
- Set out 1 set of labeled cups, 1 unlabeled cup, universal indicator, 1 long-handled spoon or other stirring equipment, 1 measuring spoon or cup.
- If distributing the pH color charts, place them with the set-ups for each group; if projecting, set up the projection.
3. Place 200 mL white vinegar in the “vinegar” cup.
4. Place 200 mL water in the “water” cup.
5. Place 100 mL milk of magnesia in the “milk of magnesia” cup.
-
Introduce Activity
Instructions
Briefly describe the goals:- You will make a color-changing vortex with universal indicator, milk of magnesia, and vinegar.
- Along the way, you will explore the properties of milk of magnesia and vinegar.
Talking Points
- What do you already know about milk of magnesia? When do you use it?
- What do you already know about vinegar? When do you use it?
- You will make a color-changing vortex with universal indicator, milk of magnesia, and vinegar.
-
Prepare the Milk of Magnesia
Instructions
Direct participants to:- Add water to the milk of magnesia cup until it is about half full.
- Add 5-10 drops of universal indicator (or 1 tsp red cabbage indicator) to provide color. Note the color.
Talking Points
- What does the milk of magnesia look like at the beginning?
- What does the color of the indicator tell you about the milk of magnesia?
-
React Milk of Magnesia with Vinegar
Instructions
Assign jobs for in each group, such as stirrer, vinegar measurer, and vinegar pourer.Direct participants to:
- Stir until a vortex (or “tornado”) forms. The stirrer will need to keep stirring throughout the activity.
- While stirring the solution, add 10 mL (1 tbls) of vinegar.
- Continue adding vinegar in 10 mL increments until the color change is permanent, and the solution turns clear.
Talking Points
- Do you observe a color change when you add the vinegar? Is the color change permanent?
- Why do you think the color change is not permanent at first?
- Why do you think the mixture became clear?
-
Discuss Results
Instructions
- Compare the amount of vinegar each group used to complete the reaction.
- Discuss students’ observations and the chemistry of what’s happening (see Explore the Chemistry)
Talking Points
- How does the pH change throughout the reaction?
- Why do you think milk of magnesia is used as an antacid?
- What is the role of equilibrium in the reaction?
- Where else might the solubility of metal hydroxides be a concern?
-
Clean Up
Instructions
- Dispose of all solids in the trash.
- Pour all remaining liquids down the drain.
- Wipe all work surfaces clean with water, or a damp cloth.
- Wash hands thoroughly.
Here are some key themes to explore with the audience once they've completed the activity. Adjust the details to match the level of your audience.
What is milk of magnesia?
Milk of magnesia is a type of mixture of Mg(OH)2 in water called a suspension. It looks cloudy because only 0.012 g Mg(OH)2 can dissolve in one liter of water at room temperature; the rest is tiny particles that can't dissolve but stay dispersed in the water.
The small amount of magnesium hydroxide that does dissolve releases enough OH- ions to create a basic solution:
Mg(OH)2(s) ⟶ Mg2+(aq) + 2 OH-(aq)
The indicator changes color to indicate that the solution is basic.
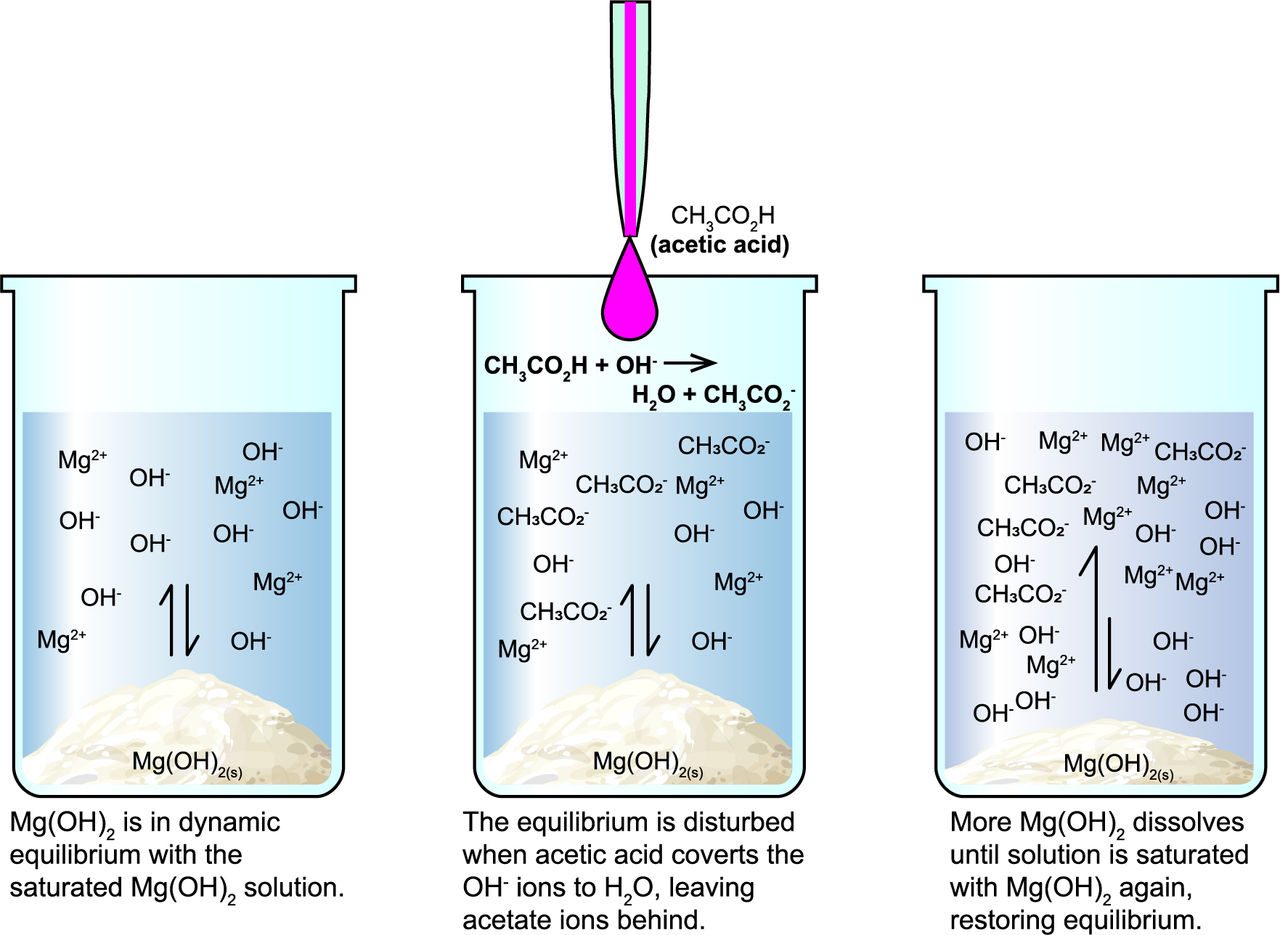
What is dynamic equilibrium?
After the water dissolves as much Mg(OH)2 as possible, the remaining solid forms a dynamic equilibrium with the solution. Individual Mg2+ and OH- ions continuously come together to form solid Mg(OH)2, while solid Mg(OH)2 dissolves, forming ions.
However, the total concentrations of Mg2 and OH- ions do not change.
What's happening with the vinegar?
Vinegar contains acetic acid (CH3CO2H). When it is added to the mixture, it neutralizes the OH- ions to form water:
Mg2+(aq) + 2 OH-(aq) + 2 CH3CO2H(aq) ⟶ Mg(CH3CO2)2(aq) + 2 H2O(l)
When a system in dynamic equilibrium is disturbed, it will shift to restore equilibrium. This is called Le Châtelier’s principle. In our system, reacting the OH- ions to form water removed them from the Mg(OH)2 equilibrium, so more Mg(OH)2 dissolves, until the OH- ion concentration is restored. The changes in acidity are again reflected in the indicator’s color changes.
As more vinegar is added, the process repeats until all of the Mg(OH)2 is dissolved, resulting in a clear solution. When all of the OH- ions are reacted, the now-acidic solution retains its final color change.
Speeding up or slowing down
You may also notice that the reaction mixture feels warm. As with many acid-base experiments, the reaction of magnesium hydroxide and vinegar generates heat. Adding ice to the system slows down the reaction, allowing more time for you to observe the color changes.
Real-world applications
Because of its ability to neutralize acid, milk of magnesia has been used to treat excess stomach acid since the early 1800s. Other bases, such as calcium carbonate (chalk) or sodium bicarbonate (baking soda) have also been popular through the years.
Le Châtelier’s principle is also why metals in drinking water can be a concern. Lead and other metals often precipitate in pipes as hydroxides. While pure water is neutral, standing water absorbs carbon dioxide from the air, forming carbonic acid. The acid then reacts with the metal hydroxides, leaching them into the water. Fortunately, this only occurs in small amounts; running your drinking water for a few seconds prior to filling your glass is usually sufficient to flush out metal build-up.
Additional use
At higher doses, milk of magnesia passes from the stomach to the intestines, where the undissolved solid can increase the bulk of the stool; the minimal Mg2+ encourages the absorption of water, which also increases bulk. Because of this, milk of magnesia can also be used to treat constipation.
References
- American Chemical Society, 2023
- ACS Student Chapter at Georgia Gwinnett College
- National Center for Biotechnology Information. "PubChem Compound Summary for CID 73981, Magnesium Hydroxide" PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Milk-of-magnesia (accessed September 2023)