Question to investigate
How can you layer three different salt water solutions so that they stay separate? Is salt water heavier or lighter than water?
Chemistry concepts
- Density describes substances based on much mass they have in a certain volume.
- The density of the salt water solution increases as the amount of salt dissolved in water increases.
- Less dense solutions float on top of solutions of greater density.
Activity logistics
- Ages: As written, this activity is suited for ages 8–12.
- Time: 30 minutes–1 hour
Be safe
- Safety goggles are required.
- Do not eat or drink any of the materials used in this activity.
- Wear gloves to prevent stain on hands.
- Cover your work area with newspaper or similar material to absorb any spills.
- Work with an adult.
- Read and follow all directions for the activity.
- Read all warning labels.
- Wear Personal Protective Equipment (PPE), such as goggles, safety glasses, or gloves.
- Tie back long hair, roll up sleeves, and secure loose clothing.
- Be sure to clean up and dispose of materials properly when you are finished with an activity.
- Wash your hands well before and after the activity.
Disposal: Dispose of all solid waste in the trash. The liquids can be disposed of down the drain with plenty of water.

What you’ll need
- 7 tsp. (about 35 mL) salt
- 1 qt. (about 1 liter) water
- 1 set of assorted food colorings
- Clear drinking straw
- 1 small test tube or narrow jar
- 4 8-ounce (about 235 mL) plastic cups
- 3 stir sticks or spoons
- Measuring spoons
Procedure
- Label each of the cups 0, 1, and 2.
- Fill each cup about half-full of water.
- Add 5 drops of food coloring to each of the labeled cups as indicated below.
- Cup 0: Red
- Cup 1: Yellow/Green
- Cup 2: Blue
- Add level teaspoons of salt to each of the labeled cups as indicated below. Stir until the salt in each cup dissolves.
- Cup 0: No salt
- Cup 1: 1 tsp.
- Cup 2: 3 tsp.
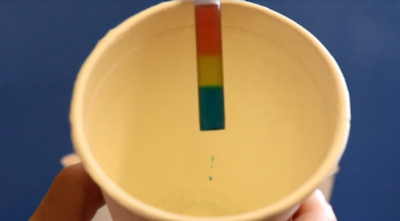
- Dip the straw about 2 cm into the red salt water solution in cup 0. Place your finger firmly over the top of the straw. Be careful not to break the seal or else you will spill the water! It may help to hold the straw over a cup so you do not spill.
- Dip the straw about 4 cm into the yellow or green salt water solution in cup 1. Remove your finger and replace it over the top of the straw.
- Dip the straw about 6 cm into the blue salt water solution in cup 2.
- Record your observations in the printable PDF.
What did you observe?
Draw or describe what you observe. Download this worksheet to record your observations.
What else can you try?
What would happen if you repeated the experiment but put the colored salt water in the test tube in a different order? Try it!
How does it work?
When you dissolve salt in water, you make a salt water solution. Adding more salt to the solution increases its density. Density describes substances based on much mass they have in a certain volume.
The more dense solutions stay on the bottom, and the less dense solutions float on top of solutions of greater density. The result is a tri-color rainbow! The rainbow effect would not form if the order were reversed. The denser solution would fall downward and contaminate the less dense solution.
This activity is adapted from an activity that originally appeared in the Celebrating Chemistry issue for Chemists Celebrate Earth Day 2018, written by Sanda Sun, Ph.D.