The Secret Science of Self-Inflating Balloons
Ages
5 - 13 years
Activity Time
Preparation: 15 mins
Activity: 15 mins
Group Size
* Participants work in pairs/trios
* 1 facilitator per 2-3 groups
ACS Student Chapter at Barry University Presents:
Self-Inflating Balloons
- Common uses of citric acid and baking soda
- Production of carbon dioxide from reaction of citric acid and baking soda
- Changes in physical properties that indicate chemical reaction
- Use of carbon dioxide to inflate a balloon
- Self-inflating balloons may squirt, especially if handled roughly.
- Potential hazards include:
- Acids & bases (citric acid, baking soda)
- Allergens (latex)
- Broken glassware
- Ignition sources (flame)
- Spills and splashes
- Conduct your own RAMP assessment prior to presenting the activity.
Each group should be provided:
- 1 tsp (5g) citric acid
- 2 tsp (~22g) baking soda
- 50 mL water
- 2 small cups
- Permanent marker
- 1 spoon/spatula
- Funnel (if desired)
- 1 flask/bottle with narrow neck, 6-8 oz or 250 mL
- 1 self-inflating balloon per student
- 1 latex balloon
- Any additional materials identified in your RAMP analysis
The following additional materials will be required if you plan to do the Flame Activity (optional):
- Small candle (tealight, votive)
- Lighter or matches
- Beaker
Prior to Activity
Customize Activity to Venue
- Review RAMP safety worksheet
- Adapt procedure to your venue and participants
- List appropriate procedures for accidents, emergencies
Identify Safety Practices
- Wear personal protective equipment (goggles, gloves, etc.)
- Secure loose hair, clothing
- Prohibit eating, drinking
- Additional practices identified in RAMP worksheet
Prepare Materials
- Prepare 10% citric acid
solution: dissolve 5 g citric acid
in 50 mL water. - Label 1 small cup “citric acid”
and the other “baking soda.” - Repeat for each group.
On-Site
For each group:
- Pour 50 mL 10% citric acid solution in labeled cup.
- Place 2 tsp. baking soda in a separate, labeled cup.
- Arrange the cups, latex balloon, spoon/spatula, funnel (if desired), flask/bottle, and 2-3 self-inflating balloons within easy reach.
For flame demo:
- Set candle, lighter, and beaker in a safe location, at least 3 m away from participants.
- Ensure area is free of flammables and that fire safety equipment is nearby.
-
Demonstrate the Self-inflating Balloon
Instructions
- Show participants your self-inflating balloon.
- Activate the balloon by breaking the citric acid pouch inside the balloon and shaking it.
- As the balloon fills with gas, explain that the participants will be investigating the chemistry that makes this possible.
Talking Points
- What is this? What do you expect to happen?
- Why did the balloon inflate?
-
Combine the Baking Soda and Citric Acid to Inflate a Latex Balloon
Instructions
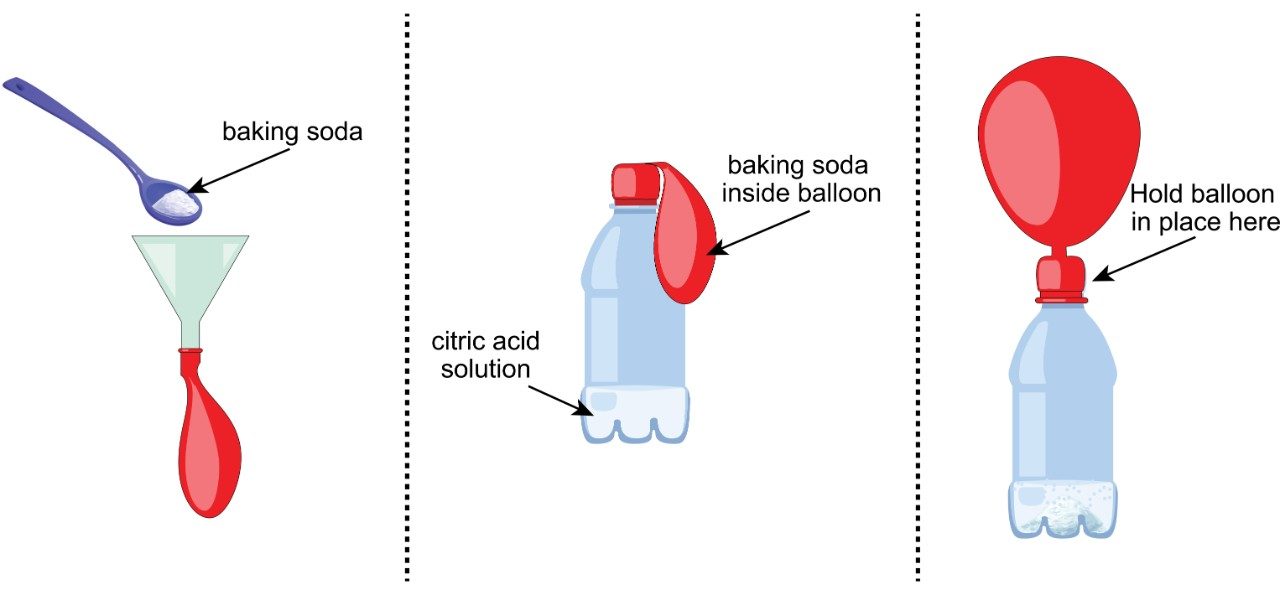
Direct participants to:- Pour citric acid solution into the flask/bottle.
- Use a funnel, spatula, or spoon to put baking soda inside the balloon.
- While keeping the baking soda in the balloon, carefully stretch the neck of the balloon over opening of the flask or bottle. The balloon will look like a floppy hat for the flask or bottle (see illustration).
- Holding the neck of the balloon firmly in place, gently lift the balloon to pour the baking soda into the citric acid solution.
- Swirl the bottle to combine. Bubbles should form and the balloon should inflate.
Talking Points
- What do you observe?
- Why is the balloon inflating? (See Explore the Chemistry for explanation.)
- Pour citric acid solution into the flask/bottle.
-
Demonstrate Effect of Carbon Dioxide on Flame
Instructions
- Light the candle.
- Pinch the neck of the balloon from one of the participants’ flask and carefully remove from bottle. Release the contents of the balloon into your beaker.
- Pour the gas from the beaker onto the flame of the candle.
Talking Points
- What does a flame need to keep burning?
- What stopped the flame from getting the oxygen it needs?
- Is carbon dioxide heavier or lighter than air?
-
Explore the Self-inflating Balloons
Instructions
Direct participants to:- Activate their self-inflating balloons.
- Younger participants may find it easier to stomp on the balloon over the citric acid packet. Older participants can be told to push on the packet like they are performing CPR.
Talking Points
- Why are the balloons inflating?
- Where else do you find citric acid and baking soda?
- Activate their self-inflating balloons.
-
Clean Up
Instructions
- Dispose of all solids in the trash.
- Pour all remaining liquids down the drain.
- Wipe all work surfaces clean with a damp cloth.
- Wash hands thoroughly.
Here are some key themes to explore with the audience once they've completed the activity. Adjust the details to match the level of your audience.
What is citric acid?
Citric acid (C6H8O7) is a compound found naturally in citrus fruit and other foods. Like all acids, it is sour, the U.S. Food and Drug Administration categorizes it as “Generally Recognized as Safe,” so it is often added to candies and drinks to produce a tart or sour taste. In it’s pure form, it is a white powder.
What is baking soda?
Baking soda, also known as sodium bicarbonate (NaHCO3), is a base. Like all carbonates, baking soda reacts with acids to produce a salt, water, and carbon dioxide gas.
What happened inside the balloon?
When citric acid and baking soda react, they form sodium citrate, carbon dioxide gas, and water.
In the latex balloon segment, the CO2 formed from reacting the citric acid and baking soda fills the balloon.
The self-inflating balloon is made of aluminum-coated polyethyene and contains a pouch of citric acid and loose baking soda. Breaking the pouch allows the citric acid and baking soda to combine. Carbon dioxide is produced, inflating the balloon.
Why did the flame go out?
In the flame segment, the CO2 from the balloon is transferred to a beaker. Because CO2 is heavier than air, it pushes the air out of the beaker and fills the beaker. Tipping the beaker over the flame pours the CO2 out, pushing away the air (and the oxygen in it) and smothering the flame.
References
- American Chemical Society, 2023
- ACS Student Chapter at Barry University
- Adapted from Kids & Chemistry React with Self-Inflating Balloons, 2018. https://www.acs.org/content/dam/acsorg/education/outreach/kids-chemistry/react-balloons-presenter-guide.pdf





