Lesson Overview for Teachers
View the video below to see what you and your students will do in this lesson.
Objective
Students will be able to plan and carry out an investigation to identify a liquid based on how it interacts on different paper surfaces. Students will also be able to explain that since different liquids are made of different atoms and molecules, they act in their own characteristic way.
Key Concepts
- When observing different liquids, the way a liquid acts on paper is a characteristic property of the liquid and can be used to identify it.
- For the test to be fair, the same amount of each liquid should be placed on the paper in the same way.
- The different atoms and molecules in a liquid interact with the paper in a characteristic way.
NGSS Alignment
- NGSS 5-PS1-1: Develop a model to describe that matter is made of particles too small to be seen.
- NGSS 5-PS1-3: Make observations and measurements to identify materials based on their properties.
Summary
- Students will test four known liquids and an unknown liquid on two different paper surfaces. They will use their observations to identify an unknown liquid.
- Students will realize that by using a combination of results from two tests, they can successfully identify an unknown liquid.
- Students will also add water and salt water to green food coloring on a coffee filter. They see a distinct difference in the way each liquid makes the colors in green food coloring separate.
Evaluation
Download the student activity sheet and distribute one per student when specified in the activity. The activity sheet will serve as the Evaluate component of the 5-E lesson plan.
Safety
Make sure you and your students wear properly fitting safety goggles. Isopropyl alcohol is flammable. Keep it away from flames or spark sources. Read and follow all warnings on the label.
Clean-up and Disposal
Remind students to wash their hands after completing the activity. Alcohol should be disposed of according to local regulations. All other common household or classroom materials can be saved or disposed of in the usual manner.
Materials for each group
- Water in cup
- Isopropyl alcohol (70%) in cup
- Detergent solution in cup
- Salt water in cup
- 1 additional small cup
- 5 droppers
- Wax paper
- Construction paper
- Pencil
Teacher preparation
Use a permanent marker to label 5 small cups Water, Salt water, Alcohol, Detergent, and Unknown for each group. The unknown is water.
Make solutions for the class according to the following procedure. These recipes make 1/4 cup of each solution, which is enough for 8 groups to conduct the activity.
- Water—Use 1/4 cup regular tap water.
- Isopropyl alcohol—Use 1/4 cup 70% isopropyl alcohol.
- Salt water—Add 2 tablespoons salt to 1/4 cup tap water.
- Detergent solution—Add 1 teaspoon of liquid hand soap or detergent and 1/4 cup water. Stir gently until well-mixed.
- Unknown – Water - Use 1/4 cup regular tap water.
Place about 1 teaspoon of each liquid into its labeled cup for each group.
Engage
1. Introduce the challenge of identifying an unknown liquid.
Show students the four liquids they will be working with: water, salt water, alcohol, and detergent solution. Tell students that since the liquids are different, they should look and act differently when placed on a surface like plastic, paper, or something else. Explain that you will also give them an unknown liquid that is the same as one of the knowns. Their job is to test the known liquids and the unknown on different surfaces to figure out what the unknown liquid is.
Give each student an Activity Sheet (PDF).
Students will record their observations and answer questions about the activity on the activity sheet.
Explore
2. As a class, develop a method for testing the liquids on the paper surfaces, starting with wax paper.
Question to investigate: Can you identify an unknown liquid by testing liquids on different surfaces?
Materials for each group
- Water in cup
- Isopropyl alcohol (70%) in cup
- Detergent solution in cup
- Salt water in cup
- Unknown in cup
- 5 droppers
- Wax paper
- Construction paper
- Pencil
Ask students:
- How should we test all four liquids and the unknown on the wax paper?
Listen to student suggestions and if necessary, guide students to the idea of using one drop of each liquid dropped onto the wax paper at the same time.

Procedure
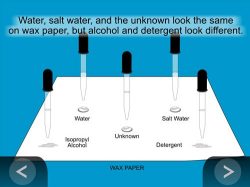
- Use a pencil to label the wax paper W, SW, A, and D for water, salt water, alcohol, and detergent. Mark the paper in the middle with U for the unknown.
- Use a dropper to get a small amount of each liquid.
- At the same time, gently squeeze 1 drop of each liquid onto its labeled area of the wax paper.
Expected results
The water, salt water, and unknown beaded up on the wax paper. The alcohol and detergent solution spread out.
Ask students:
- What did you notice?
The water and salt water beaded up and the alcohol and detergent flattened out. The unknown beaded up like the water and salt water. - What two liquids can you eliminate that are not the unknown?
Alcohol and detergent solution acted differently from the unknown so the unknown is not alcohol or detergent solution. - What must be the identity of the unknown?
The unknown must be either water or salt water.

3. Have students test the water, salt water, and the unknown on construction paper.
- Use a pencil to label the construction paper W and SW for water and salt water and U for the unknown.
- Use a dropper to get a small amount of each liquid.
- At the same time, gently squeeze 1 drop of each liquid onto its labeled area of the construction paper.
Expected results
The water soaked into the construction paper more than the salt water, which stayed on top of the paper in a bead longer than the water. The unknown soaked in similarly to the water.
Ask students:
- What did you notice?
The water absorbed into the construction
paper more than the salt water did. The unknown absorbed into the construction paper like the water.
- What is the identity of the unknown?
Water
Explain
4. Show an animation to explain that the liquids are made from different atoms and molecules, so they act differently when tested on the paper surfaces.
Show the Animation Identifying an Unknown Liquid.
Explain that each liquid is made up of different molecules. The molecules have different characteristics, and they interact with the wax paper and construction paper in their own unique way.
Extend
5. Have students test water and salt water with green food coloring on a coffee filter.
Tell students that since water and salt water are different, they may act differently when mixed with other substances. Tell students that they will do a test to see how water and salt water interact with green food coloring.
Note: Students will do a form of chromatography on green food coloring using the different liquids they have been investigating. Using only water and salt water is the simplest version of this EXTEND. If you want, you could have students use all four liquids on four different coffee filters. The results should all look different.
Materials for each group
- 2 white coffee filters
- 2 cups
- 3 or 4 drops of green food coloring in a small cup
- 2 cotton swabs
- Water
- Salt water
Procedure
- Use a pencil to mark the coffee filters W and SW, for water and salt water.
- Dip your cotton swab into the food coloring and then use the swab to make a dark dot in the center of both coffee filters.
- Dip one end of a clean cotton swab into the water and move it around a bit to be sure it gets wet. Place the wet end on the dot on the coffee filter labeled W as shown.
- Use a different cotton swab and dip it into the salt water and move it around a bit to be sure it gets wet. Place the wet end on the dot on the coffee filter labeled SW as shown.1.
- If it looks like the color is not spreading much, re-dip the cotton swab into the water and salt water and put the wet end on the color again.

Water Salt water
Expected results
The different liquids will interact with the green food coloring differently.
The water causes all of the color to spread away from the center, leaving an area of white with a ring of yellow and then a ring of blue around the yellow.
The salt water will have a round area of yellow in the center and a rim of blue around it.

Water Salt water
Explain
6. Explain why water and salt water interact with the food coloring differently.
The water interacts with the food coloring resulting in the yellow and blue moving about the same distance. The salt water must interact with the food coloring differently.
The experiment shows that since the liquids contain different molecules, they interact with the food coloring differently.
Note: If you want students to think about the results in more detail, you could ask students what they think it is about the salt water that gave the different results. You could suggest that maybe the ions in salt are more attracted to the yellow food coloring and to the filter so the yellow doesn’t travel as far as the blue.





