What molecule am I?

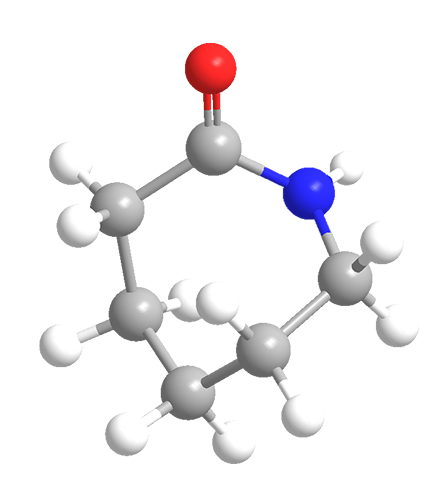
ε-Caprolactam is a crystalline cyclic amide with a melting point of 70 °C. It is soluble in water, most oxygenated and chlorinated solvents, and some hydrocarbons.
Because ε-caprolactam is the only common caprolactam isomer, the ε is usually dropped. It derives its name from ε-aminocaproic acid, or 6-aminohexanoic acid; in principle, the lactam is formed when the terminal carboxylic acid and amino groups react to form the amide.
The amide formation reaction succeeds only when run in dilute solution; otherwise, aminocaproic acid polymerizes (which is a good thing; see below). The commercial synthesis consists of the acid-catalyzed Beckmann rearrangement of cyclohexanone oxime, which was discovered by Prussian chemist and Nobel Prize winner Otto Wallach way back in 1900. Numerous articles and patents have been devoted to improving this method ever since.
Wallach didn’t live to see it, but caprolactam turned out to be extremely valuable. In 1938, Paul Schlack at IG Farben found that heating caprolactam neat to 260 °C causes the ring to open and the terminal functional groups to react to form a long-chain polyamide.
This polymer later became known as “nylon 6”*. It can be formed into high-strength fibers, resins, and films that have dozens of end-use applications ranging from clothing to violin strings to automotive mechanical parts.
*Nylon 6 is similar to—but should not be confused with—nylon 6,6, which is prepared from hexamethylenediamine and adipic acid.
MOTW update:
April 02, 2018
Last week, C&EN reported that potentially harmful volatile organic compounds (VOCs) can be emitted from the plastics used in 3-D printers. Among the VOCs are former Molecules of the Week styrene (June 8, 2015), formaldehyde (May 29, 2006), and caprolactam (September 19, 2016). Regulatory standards may be in the offing for manufacturers of these printers.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

