What molecule am I?

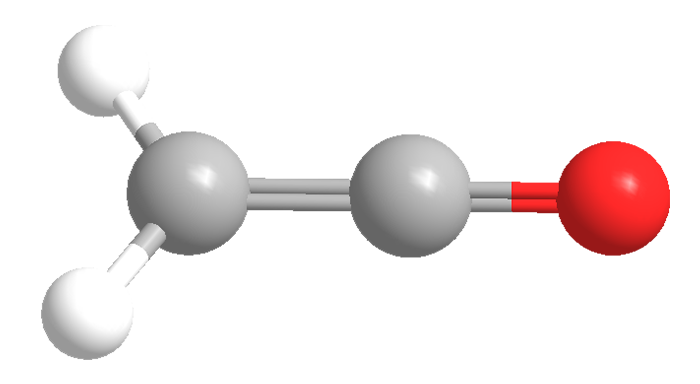
Ketene (systematic name ethenone) is a colorless, toxic gas with a “penetrating” odor, according to the Merck Index. It is soluble in essentially all organic solvents, but it decomposes in water to form acetic acid. Its tautomer, ethynol, is known to exist only by photoisomerizing ketene in an argon matrix. Nobel Prize–winning German chemist Hermann Staudinger discovered the ketene family of organic compounds early in the 20th century.
Ketene is prepared by pyrolyzing acetone, acetic acid, or acetic anhydride or by treating acetyl chloride with a nonprotic nucleophile. It is useful for acetylating nucleophiles to make esters, amides, and other compounds that cannot easily be made with other reagents. Ketenes were used in the synthesis of the early antibiotics penicillin and amoxicillin.
The anions cyanate (NCO–) and 2-phosphaethynolate (PCO–) are isoelectronic with ketene. Now Alexander Hinz and Jose M. Goicoechea at Oxford University (UK) report the preparation of the arsenic analogue, 2-arsaethynolate (AsCO–) via a three step sequence that starts from elemental arsenic. AsCO– readily reacts with ketenes and carbodiimides to form 4-membered arsenic heterocycles.
MOTW update:
October 17, 2022
Ketene1 is a colorless, toxic gas that is useful in organic synthesis. This month, Guangjin Hou, Xiulian Pan, Xinhe Bao, and co-workers at the Chinese Academy of Sciences (Dalian) described the chemistry of converting ketene to gasoline-range hydrocarbons by passing it over H-SAPO-11 molecular sieves2 at temperatures up to 400 °C.
1. CAS Reg. No. 463-51-4.
2. H-SAPO-11 is an acidified medium-pore silicoaluminophosphate molecular sieve, CAS Reg. No. 308075-33-4.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

