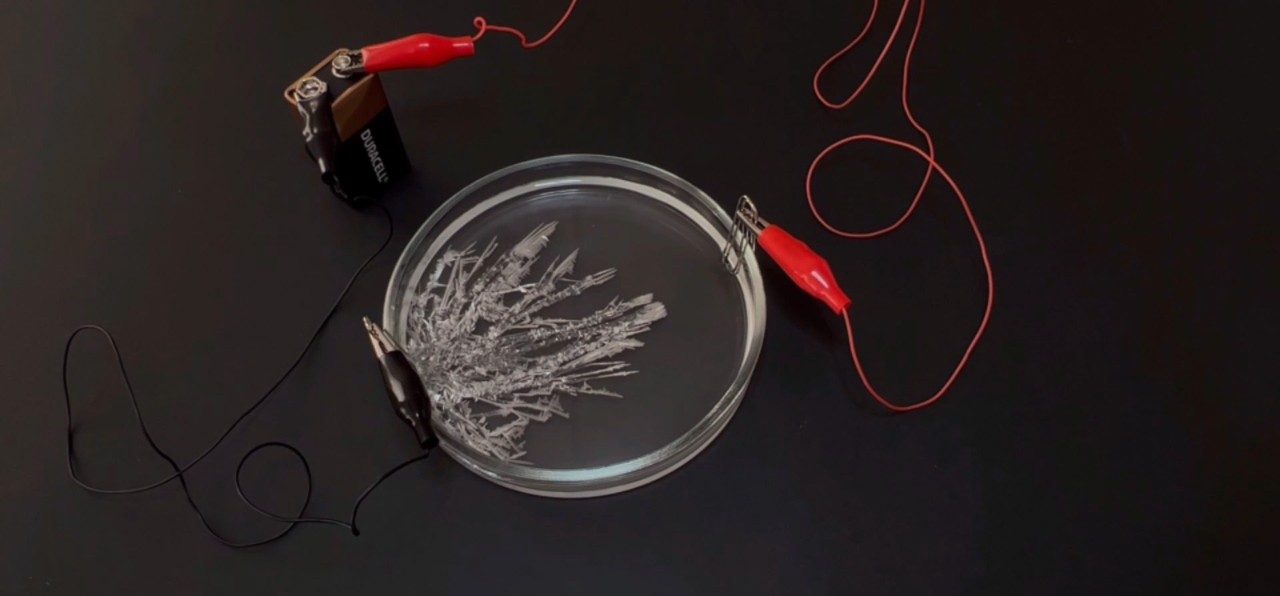
Tin Crystals
Ages
12 - 18 Years
Activity Time
Prep: 15 mins
Activity: 10 mins hands-on, 24 hr total
Group Size
25-250 observers
ACS International Student Chapter at San Diego State University-Georgia Presents: Tin Crystals
- Disproportionation reactions: SnCl2 forms both SnCl4 and tin metal
- Redox reactions: SnCl2 loses electrons (is oxidized) to form SnCl4 and gains electrons (is reduced) to form Sn
- Electrolysis: Oxidation of SnCl2 takes place at the anode and reduction of SnCl2 takes place at the cathode
- Best done in a laboratory setting or venue in which presenters and spectators are separated by at least10 feet.
- Complete reaction takes 24 hours; you may wish to have a completed version available to show the audience..
- Work in a well-ventilated area.
- Potential hazards include:
- Acids
- Sensitizers
- Broken glassware
- Inhalation hazards
- Oxidizers
- Spills and splashes
- Conduct your own RAMP assessment prior to presenting the activity.
- 250 mL beaker
- Glass stirring rod
- 2 small paper clips
- 9-V battery
- Petri dish
- 2 wires with alligator clips on either end, or battery cap with alligator clip leads
- 5 g tin(II) chloride, SnCl2
- 50 mL 12 M HCl solution
- 1-2 cm copper or steel wire
Optional
- Overhead projector
- Clear acetate film
OR
- Camera connected to a projection system
- White paper
- Any additional materials identified in your RAMP analysis
Prior to Activity
Customize Activity to Venue
- Work in a well-ventilated area.
- Revise procedure to adapt to your specific venue and participants.
- List appropriate procedures for accidents, emergencies.
Identify Safety Practices
- Wear appropriate personal
protective equipment (e.g.,
goggles, gloves, etc.). - Secure loose hair, clothing.
- Prohibit eating, drinking.
- Clean work area, wash hands after activity.
- Ensure a minimum of 10 feet between presenters and audience.
Prepare Materials
- Place 5 g SnCl2 in beaker.
- Add 50 mL HCl solution and stir to dissolve.
On-Site
- Clip paper clips to opposite sides of the petri dish, with longer ends on the inside, nearly touching the bottom.
- Place the petri dish on a piece of white paper for easier viewing; if using an overhead projector, place the petri dish on a clear, colorless acetate slide on the projector.
If desired, annotate the paper or acetate slide with the reactions described in the Explore the Chemistry section.
-
Introduce the Tin Solution
Instructions
- Show tin solution to participants.
- Explain that you will be creating tin metal crystals.
Talking Points
- What do you expect tin to look like?
- How does your expectation compare with the tin chloride solution?
-
Begin Electrolysis
Instructions
- Transfer tin solution to petri dish.
- Attach two wires to the paper clips using the alligator clips. Connect the other ends of the wires or the battery cap to the 9-V battery.
- Optional: Place a piece of copper or steel wire in the petri dish, with the ends pointed at the paper clips.
Talking Points
- What do you observe in the solution near the paper clips?
- What role does each part of the set-up play in an electrical circuit?
- What do you think is happening in the petri dish?
-
Conclude Electrolysis
Instructions
- Allow current to run up to 24 hours. In the meantime, show the finished version of the electrolysis
- Explain how tin disproportionates to create the crystals (see Explore the Chemistry).
Talking Points
- What do you observe in the petri dish?
- What happens if you reverse the reconnect the alligator clips to opposite paper clips?
-
Clean Up
Instructions
- Neutralize solutions with sodium bicarbonate and dispose according to local regulations.
- Clean and dry equipment with detergent and water.
- Wash hands thoroughly.
Here are some key themes to explore with the audience once the activity is complete. Adjust the details to match the level of your audience.
The formation of tin crystals from the tin(II) chloride solution is an example of electrolysis. In electrolysis, an electric current induces a chemical reaction in which reactants are reduced (gains electrons) and oxidized (loses electrons), or a redox reaction.
In this activity, the SnCl2 completely dissociates in water to form Sn2+ and Cl- ions. These ions (plus the ions from the acid) create an electrolyte solution, or a solution capable of carrying a current. The battery powers a current that runs through the circuit created by the battery, connecting wires, paper clips, and electrolyte solution.
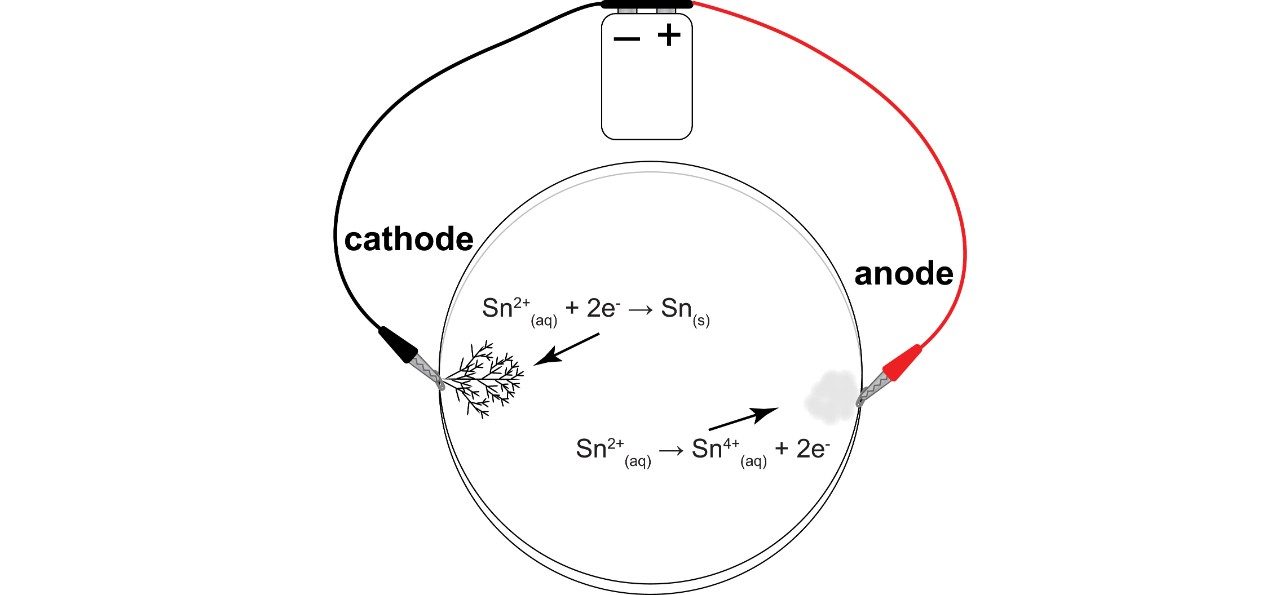
In the petri dish, the current drives a disproportionation reaction. Disproportionation is a type of redox reaction in which a single reactant iform both oxidized and reduced products.
Reduction takes place at the cathode, where half of the tin(II) chloride gains two electrons to become tin metal. This half of the reaction can be written as:
Sn2+(aq) + 2e- → Sn(s)
(Cl- ions are not shown here because they neither gain nor lose elections in this process.)
Oxidization takes place at the anode, where the half of the tin(II) chloride loses two electrons to become tin(IV) chloride. This half of the reaction can be written as:
Sn2+(aq) → Sn4+(aq) + 2e-
Tin(IV) chloride is insoluble and the white precipitate you see at the anode.
Sn4+ (aq) + 4Cl- → SnCl4(s)
The overall reaction is:
2Sn2+(aq) + 4Cl-(aq) → SnCl4(s) + Sn(s)
If you added a piece of wire to the petri dish, it forms an additional electrode, with the oxidation and reduction reactions repeating at either end.
Electrolysis is a common technique for purifying metals and coating surfaces. It is also used to split water (H2O) into hydrogen and oxygen gases for clean energy sources and is researched for other green applications.
References
- American Chemical Society, 2023
- ACS International Student Chapter at San Diego State University–Georgia