Lesson Overview for Teachers
View the video below to see what you and your students will do in this lesson.
Objective
Students will be able to develop and explain a particle-level model to describe their observations of water dissolving the colored sugar coating of an M&M. Students will be able to use this model to develop an explanation for their observations of an M&M in a sugar solution.
Key Concepts
- Dissolving a solid in a liquid depends on the interaction between the particles of the liquid (solvent) with the particles of the solid (solute).
- Dissolving happens when the attraction between the particles of the solvent and solute are strong enough to overcome the attraction of the particles of the solute for each other.
NGSS Alignment
- NGSS 5-PS1-1: Develop a model to describe that matter is made of particles too small to be seen.
Summary
So far, students have seen how the attraction and arrangement of particles are different in a solid, liquid, and gas.
In this lesson:
- Students explore the interaction of two substances and see that they can use what they have learned about the interaction of particles to explain their observations.
- Students place an M&M in water and see the colored sugar coating dissolve around the M&M.
- Students help develop a model to explain that the attraction of water molecules for sugar and color (dye) molecules is a good explanation for why the sugar coating dissolves.
- Students then test whether the coating dissolves as well in a sugar solution as it does in plain water.
- Students then put three or four different colored M&Ms together in water and watch the coatings dissolve. Students will see a distinct “line” where the colors meet.
- Students use molecular models to make an argument about why the dissolving M&Ms form a line.
Evaluation
Download the student activity sheet and distribute one per student when specified in the activity. The activity sheet will serve as the Evaluate component of the 5-E lesson plan.
Safety
Make sure you and your students wear properly fitting safety glasses or goggles.
Clean-up and Disposal
Remind students to wash their hands after completing the activity.
All common household or classroom materials can be saved or disposed of in the usual manner.
Materials for each group
- Different colored M&Ms
- 2 small white plastic plates
- Sugar
- Tablespoon
- Cup
- Room-temperature water
- Plastic “waste” container
- Paper towels

Engage
1. Have students look at the colored candy coating of an M&M.
Ask students to look at an M&M and have a discussion about what they think the coating is made from. Let students know that the candy coating is mostly sugar with some coloring. Ask students if they think the coating will dissolve in water.
Give each student an Activity Sheet (PDF).
Students will record their observations and answer questions about the activity on the activity sheet.
Explore
2. Have students place one M&M in room-temperature water.
Question to investigate: What happens to the sugar and color coating of an M&M when you put it in water?
Materials for each group
- M&Ms (different colors)
- Room-temperature water
- 2 small white plastic plates

Procedure
- Place enough water in the plate so that it will cover an M&M.
- Place 1 M&M in the center of the plate. Do not swirl or stir.
- Observe for about 2 minutes.
Ask students:
- What did you observe?
The color coating (coloring and sugar) dissolved from the M&M and formed an area of color around it.
Explain
3. Discuss why the water was able to dissolve the coating of the M&M.
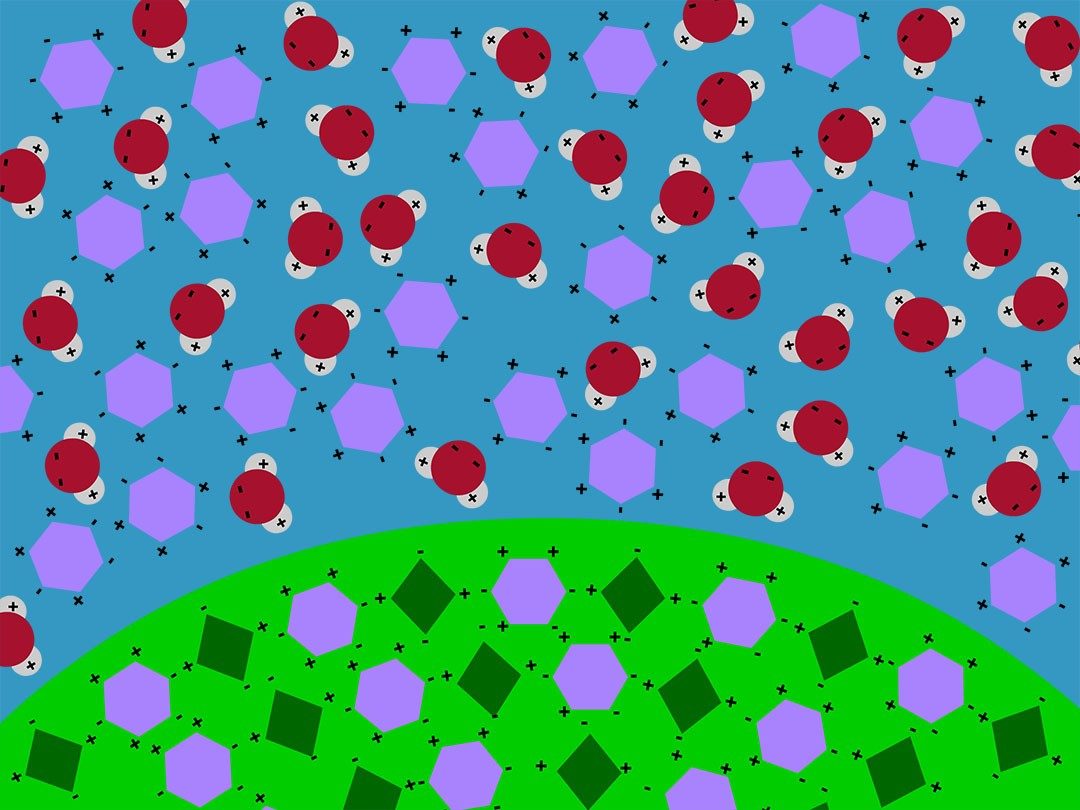
Show the animation Dissolving an M&M.
Explain that water molecules interacted with sugar and coloring molecules in the color coating of the M&M and caused the coating to dissolve in the water. Explain that the water molecules are shown as one oxygen atom (red) attached to two hydrogen atoms (white). The sugar and the food coloring molecules are shown as simple shapes. The water molecules have areas of positive and negative charge. The sugar and food coloring molecules also have areas of positive and negative charge. Water molecules attract both sugar and food coloring molecules because positive and negative charges attract each other.
Have students dump out the water and the M&M into a plastic waste container and use a paper towel to clean and dry the plate.
4. Discuss how to design a test to see if an M&M coating dissolves as well in a sugar solution as it does in water.
Question to investigate: Will an M&M’s coating dissolve as well in a sugar solution as it does in plain water?
Materials for each group
- 2 small plastic plates
- 2 M&Ms
- Sugar
- Tablespoon
- Cup
- Room-temperature water
Ask students:
- How should we set up this experiment?
Keep everything the same except for one sample of water and one sample of sugar solution - Should we use a sample of fresh water and a sugar solution?
Yes - Should we use the same amount of each?
Yes - Should the water and sugar solution be at the same temperature?
Yes - Should we put the same color M&M into each solution?
Yes - Should we put them in at the same time and in the same place in each plate?
Yes
The idea is to have students realize that everything about the test should be the same except that one liquid is water and the other is a sugar solution. The goal is to see if the presence of sugar in the water affects how the M&M coating dissolves. Therefore, the only difference should be the sugar in the water in one of the samples.
5. Have students set up and run the experiment.

Procedure
- Make a sugar solution by dissolving 1 tablespoon of sugar in ¼ cup of water.
- Put the sugar solution in one plate and an equal amount of fresh water in a second plate.
- At the same time, place one M&M in the center of each plate. The M&Ms should be the same color.
- Observe the M&Ms for 1 to 2 minutes.
Expected results
The M&M coating does not dissolve as well in the sugar solution as it does in plain (fresh) water.
Ask students:
- What did you notice?
More of the coating dissolved and spread out in the water than in the sugar solution.
Explain
6. Discuss why there was a difference between the M&M dissolving in water and the M&M dissolving in the sugar solution.
Ask students:
- If you think about the water and sugar molecules in the sugar solution, can you think of a reason why the sugar solution doesn’t dissolve the M&M as well as fresh water does?
Show the Illustration M&M in Sugar Solution (JPG).
Explain that there are different possible explanations:
- Maybe a lot of water molecules are already attracted to sugar molecules in the sugar solution, so there aren’t as many water molecules available to dissolve the sugar from the M&M.
- Maybe there is so much sugar in the sugar solution that even when water molecules dissolve the coating, it can’t push the sugar solution out of the way, so it’s harder to spread out.
Extend
7. Have students test what happens when two or more M&Ms are on the plate at the same time.
Tell students that they can see what happens when two or more M&Ms dissolve near each other at the same time.
Question to investigate: What happens when two or more M&Ms are in the same plate of water at the same time?

Procedure
- Place enough water in a plate to cover an M&M.
- Place 3 or 4 M&Ms of different colors near each other in the water as shown.
- Observe for 2 to 3 minutes.
Ask students:
- What do you observe?
The colors spread out from the M&M, but when the colors meet, they do not mix. Instead they form a kind of “line.”
Ask students:
- Based on what you saw when you tried to dissolve an M&M in a sugar solution, why do you think the colors form a kind of “line” when they meet?
Explain that maybe the dissolved sugar from the M&M can move easily in water but it’s harder to move against another sugar solution coming off another M&M. That’s why a line forms where the sugar from two M&Ms meet.