Lesson Overview for Teachers
View the video below to see what you and your students will do in this lesson.
Objective
Students will be able to plan and carry out an investigation to compare the amount of gas produced in reactions between baking soda and baking powder when vinegar is added. Students will be able to explain that mixing substances can cause a chemical reaction, which results in the formation of a new substance. Students will also be able to explain that substances react in characteristic ways and that the way a substance reacts can be used to identify the substance.
Key Concepts
- Mixing substances can result in a chemical reaction, which produces new substances.
- Substances have characteristic chemical reactions that can be used to identify them.
- Designing a fair test to study chemical reactions requires keeping everything the same except for the one thing you want to know about.
NGSS Alignment
- NGSS 5-PS1-3: Make observations and measurements to identify materials based on their properties.
- NGSS 5-PS1-1: Develop a model to describe that matter is made of particles too small to be seen.
- NGSS 5-PS1-4: Conduct an investigation to determine whether the mixing of two or more substances results in new substances.
Summary
In this lesson, students will discover that substances have characteristic chemical reactions with other substances and that these reactions can be used to identify a substance.
- Students are shown samples of baking soda and baking powder.
- Students help design an experiment to test whether baking soda and baking powder, similar-looking powders, are made up of the same chemical.
- Students will observe that baking soda produces more bubbles than baking powder when mixed with vinegar, which is evidence that the two substances are chemically different.
- Students conclude that mixing substances can result in a reaction that produces new substances, and that the way different substances react is a characteristic property that can be used to identify a substance.
Evaluation
Download the student activity sheet (PDF) and distribute one per student when specified in the activity. The activity sheet will serve as the Evaluate component of the 5-E lesson plan.
Safety
Make sure you and your students wear properly fitting safety goggles. Vinegar may irritate the skin and cause severe eye irritation.
Clean-up and Disposal
Remind students to wash their hands after completing the activity. All common household or classroom materials can be saved or disposed of in the usual manner.
Teacher Preparation
Note: This activity works best if you make your own “baking powder” by mixing cream of tartar, baking soda, and cornstarch.
- Make a baking powder mixture by placing 1 tablespoon of cream of tartar, 1 tablespoon of baking soda, and 1 tablespoon of cornstarch in a cup. Use a popsicle stick or plastic spoon to thoroughly mix the powders.
- For each group of students, label two plastic cups Baking Soda, and two other cups Baking Powder. Place 1 teaspoon of baking soda in one labeled cup and 1 teaspoon of baking powder in another labeled cup. These will serve as source cups from which students will transfer the powders into the other empty labeled cups.
- Label three cups Vinegar for each group. Place 2 tablespoons of vinegar in one of the labeled cups. This will serve as the source cup for vinegar from which students will transfer the liquid into the other two empty labeled cups.
- Make a detergent solution by adding 2 tablespoons of water and 1 teaspoon dishwashing liquid to a cup. Label a separate Detergent Solution cup for each group of students and transfer 1 teaspoon of this detergent solution into each cup.
Materials
Materials for each group
- Vinegar (in labeled cup)
- 2 empty cups labeled Vinegar
- Baking soda (in labeled cup)
- 1 empty cup labeled Baking Soda
- Baking powder (in labeled cup)
- 1 empty cup labeled Baking Powder
- 1 teaspoon of detergent solution (in labeled cup)
- Measuring spoon, ½ teaspoon size
- Teaspoon
- Dropper
Materials for the ENGAGE demonstration
- Baking soda
- Baking powder
- 2 clear plastic cups
- Popsicle stick or plastic spoon
Materials for the EXTEND demonstration
- Graduated cylinder, 50-mL size, or plastic tablespoons
- 2 plastic teaspoons (or small measuring spoons, such as ½ teaspoon size)
- 2 empty disposable water bottles, 8-oz size
- 2 balloons (blow them up a couple times before the demonstration so they will be flexible and expand more easily)
- Vinegar

Engage
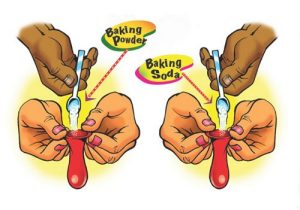
1. Show students samples of baking soda and baking powder and ask students what could be done to tell them apart.
Show students samples of baking soda and baking powder. Point out that even though the two white powders look similar, there may be differences between them. Ask students if they can suggest a test or property of the solids that might be used to show that they have different characteristics. Suggest that both powders should be tested in the same way.
Ask students:
- Could we add something to the baking soda and baking powder to compare how they react?
If students don’t suggest a test, remind them they may have seen examples of vinegar reacting with baking soda to produce bubbles, such as in the popular “volcano” demonstration. Maybe they can add vinegar to both solids (baking soda and baking powder) and compare the way they react.
Give each student an Activity Sheet (PDF).
Students will record their observations and answer questions about the activity on the activity sheet.
Explore
2. Help students design an experiment to compare the amount of gas produced when baking soda and baking powder react with vinegar.
Question to investigate: Will baking powder or baking soda produce more gas when vinegar is added?
Lead a discussion to help students design a test to compare the reactions of baking soda and baking powder with vinegar. Ask leading questions to encourage students to realize that to determine whether the reactions of the two powders are different the baking soda and baking powder must be tested in the same way.
Ask students:
- Should we use the same amount of baking soda and baking powder in the test?
Yes - Should we add the same amount of vinegar to the baking soda and to the baking powder?
Yes - Is it important to add the vinegar at the same time to the two separate cups containing baking soda and baking powder? Why or why not?
Testing the two reaction side-by-side, by adding vinegar to the two cups at the same time, makes it easier to compare not only how much bubbling is produced, but also how fast the bubbles are produced. Alternatively, you could also mark the level of bubbles produced in each cup and time how fast it takes the bubbles to rise in each cup.
Materials for each group
- Vinegar (in labeled cup)
- 2 empty cups labeled Vinegar
- Baking soda (in labeled cup)
- 1 empty cup labeled Baking Soda
- Baking powder (in labeled cup)
- 1 empty cup labeled Baking Powder
- 1 teaspoon of detergent solution (in labeled cup)
- Measuring spoon, ½ teaspoon size
- Teaspoon
- Dropper

Procedure
- Place ½ teaspoon of baking soda and ½ teaspoon of baking powder into their labeled cups.
- Add 2 teaspoons (10 milliliters) of vinegar to each of the two empty cups labeled Vinegar.
- Using a dropper, add 1 drop of detergent solution to the vinegar in each cup. Gently swirl to mix.
- Pour vinegar from both cups into the baking soda and the baking powder at the same time. Compare how the two solids react with vinegar.
Expected results:
The baking soda reacts faster and produces more bubbles than the baking powder.
Ask students:
- What did you observe?
The baking soda reacted faster with vinegar than baking powder did and also produced more bubbles. The baking powder also bubbled when vinegar was added, but the overall reaction was slower and the bubbles did not rise as high in the cup as they did with baking soda.
Explain
3. Show an animation to help explain why baking soda produces more gas than baking powder when each is mixed with vinegar.

Show the animation Vinegar Test: Baking Soda vs. Baking Powder.
Tell students that baking powder is a mixture containing baking soda along with two other ingredients. Explain that the bubbles the students observed were produced by carbon dioxide gas generated from the reaction with baking soda, a chemical in both of the powders. The other two ingredients in baking powder do not react with vinegar.
Explain that there is more baking soda in ¼ teaspoon of baking soda than there is in ¼ teaspoon of baking powder because baking powder also contains the other ingredients. So if you add vinegar to equal amounts of baking powder and baking soda, the baking soda produces more bubbles.
Extend
4. Do a demonstration to see whether baking soda or baking powder mixed with vinegar is better at inflating a balloon.
Tell students that another way they can determine whether baking soda or baking powder produces more gas when mixed with vinegar is by trapping the gas in a balloon.
Materials for the demonstration
- 2 empty disposable water bottles, 8-oz size
- 2 balloons (blow the balloons up a couple times before the demonstration so they will be flexible and expand more easily.)
- Vinegar
- Baking soda
- Baking powder
- Graduated cylinder, 50-mL size, or plastic tablespoon
- 2 plastic teaspoons (or small measuring spoons, such as ¼ - teaspoon size)
Procedure
- Make labels so students can easily tell which balloon has the baking soda and which has the baking powder.
- Add 30 mL (or 2 tablespoons) of vinegar to each bottle. Do this in front of students or tell them that you added the same amount of vinegar to both bottles.
- Have a student help you stretch the opening of the balloon so it will be easier to add the baking soda to it. Add 1 teaspoon of baking soda to the balloon. You might need to use a funnel or a smaller measuring spoon, like a ¼-teaspoon size. If using a measuring spoon, make sure the total amount of powder added is 1 teaspoon.
- Repeat step 2 for the baking powder.
- Stretch the opening of one balloon over the mouth of each bottle so that the part of the balloon containing the powder hangs down the side of the bottle.

- When you are ready, lift the two balloons at the same time and shake them a little so that the powders fall into the vinegar in the bottom of the bottles.
- Give both bottles a quick swirl and then let them sit on the table. Observe what happens.
Expected results:
The balloon with the baking soda will inflate more than the balloon with the baking powder.
Ask students:
- Is this the result that you expected?
Yes





