Lesson Overview for Teachers
View the video below to see what you and your students will do in this lesson.
Objective
Students will be able to explain that if they mix baking soda with two different substances in separate containers and observe different signs of chemical reactions, it must be because the two substances are different. The substances must be made from different molecules, which react differently with baking soda.
Key Concepts
- Because substances are made up of different atoms and molecules, they react in characteristic ways.
- Production of a gas, color changes, a change in temperature, and the formation of a precipitate are all evidence of a chemical reaction.
NGSS Alignment
- NGSS 5-PS1-4: Conduct an investigation to determine whether the mixing of two or more substances results in new substances.
Summary
In working with chemical reactions, students have seen a gas produced, a precipitate formed, and changes in color. They have also seen that substances interact in characteristic ways and that these reactions can help identify a substance. In this activity:
- Students conduct a reaction with citric acid and baking soda in a universal indicator solution. The resulting chemical reaction produces a gas, causes a color change with an indicator, and results in a decrease in temperature.
- Students then carry out a second reaction with calcium chloride and baking soda in the universal indicator solution. In this example, students observe the production of both a gas and a solid, a color change with the indicator, and a slight increase in temperature.
- Students reason that since baking soda was reacted with two different substances, it makes sense that the reactions they observed were different. Finally, students conclude that different substances have characteristic chemical reactions and that these reactions can be used to identify a substance.
Evaluation
Download the student activity sheet (PDF) and distribute one per student when specified in the activity. The activity sheet will serve as the Evaluate component of the 5-E lesson plan.
Safety
Make sure you and your students wear properly fitting safety goggles. Calcium chloride may cause serious eye damage or irritation. Citric acid is also an eye irritant. Do not handle the solids with bare hands. Universal indicator is an alcohol-based solution and is flammable. Keep away from all potential sources of ignition. Read and follow all safety warnings on the labels for all chemicals used in this activity.
Clean-up and Disposal
Remind students to wash their hands after completing the activity. At the end of the lesson, have students pour their used or leftover solutions into a waste container. Dispose of these solutions down the drain or according to local regulations. Any leftover solid citric acid or calcium chloride may be rinsed down the drain with plenty of running water.
All other common household or classroom materials can be saved or disposed of in the usual manner.
Materials
Materials needed for each group
- Citric acid
- Baking soda
- Calcium chloride
- Universal indicator
- Water
- 2 Thermometers
- Plastic measuring spoon, ½-teaspoon size
- 2 Clear plastic cups
- 2 Small cups
- Graduated cylinder or plastic tablespoon (measuring spoon)
Notes about the materials
The bulb of the thermometer must be completely submerged in the indicator solution to get an accurate reading. If your thermometers have a plastic backing that extends below the bulb, you may be able to clip the plastic backing so that it is even with the bottom of the bulb, allowing the bulb to be lower in the liquid.
Calcium chloride is available from chemical suppliers used by your school or district. The activity works well with anhydrous calcium chloride. Keep the container tightly closed to prevent the material from absorbing moisture from the air.
Universal indicator solution is also available from chemical suppliers and comes with color charts to indicate the pH of the solution.
Teacher Preparation
Label 3 small cups Citric Acid, Calcium Chloride, and Baking Soda.
Place about ½ teaspoon of citric acid, ½ teaspoon of calcium chloride, and about 1 teaspoon of baking soda in their labeled cups for each group.
Make the indicator solution for student groups:
Prepare a dilute universal indicator solution by combining 325 mL of water with 25 mL of universal indicator solution. This quantity should be enough for 10 groups of students.
Pour 30 mL of the diluted universal indicator solution into a clean plastic cup for each student group. You will also need 50 mL of the diluted indicator solution for the demonstration in the EXTEND section.
Note: Your local tap water is likely fine for the demonstration and activities in this lesson. If the diluted universal indicator solution is not green, this means that your water is either slightly acidic or slightly basic. If this happens, use distilled water (available in supermarkets and pharmacies) to dilute the universal indicator.
Engage
1. Have a discussion with students about whether they think a reaction between citric acid and baking soda should be the same or different than a reaction between calcium chloride and baking soda. Why?
Tell students that they will carry out two reactions with two sets of substances in a solution containing a pH indicator to observe and compare any changes that take place. Tell students that one reaction will be between citric acid and baking soda, while the other will be between calcium chloride and baking soda.
Remind students that they have seen reactions in which baking soda reacted with different acids to produce a gas. They have also seen reactions in which an acid or a base reacted to change the color of a pH indicator solution.
Ask students:
- Do you think that the reactions you will carry out may produce a gas?
Yes. At least one reaction (between citric acid and baking soda) should produce a gas. - Do you think the reactions you will carry out may produce color changes in indicator?
Yes. At least one (between citric acid and baking soda) will change color. - Do you think the reactions involving the two sets of substances will be the same or different?
Different because the baking soda is reacting with citric acid in one reaction and with calcium chloride in the other. Since those substances are different, their chemical reactions with baking soda should also be different.
If necessary, review with students how to read the Celsius scale of the thermometer. Explain that the bulb of the thermometer should be submerged during the reaction and when students are reading the thermometer.
Give each student an Activity Sheet (PDF).
Students will record their observations and answer questions about the activity on the activity sheet.
Explore
2. Have students make detailed observations as they carry out two separate reactions, between citric acid and baking soda and then between calcium chloride and baking soda.
Question to investigate: What are the similarities and differences in the reaction between citric acid and baking soda, and in the reaction between calcium chloride and baking soda?

Procedure
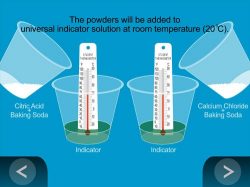
- Using a graduated cylinder or a measuring spoon, measure 15 mL (one tablespoon) of universal indicator solution and pour it into one of the clear plastic cups. Repeat this step for the other clear plastic cup.
- Place a thermometer in the indicator solution in each cup and record the initial temperature on the activity sheet.
- In a separate small cup, combine 1/2 teaspoon of citric acid and 1/2 teaspoon of baking soda.
- In another small cup, combine 1/2 teaspoon of calcium chloride and 1/2 teaspoon of baking soda.
- While the thermometers are still in the cups of indicator solution, pour the citric acid and baking soda mixture into one of the cups of indicator solution. Observe any changes that occur.
Expected results
Citric acid and baking soda
Lots of bubbles will be produced very quickly. The solution will first turn reddish-orange and then orange and the temperature will decrease.

Procedure
- With the thermometer still in the second cup of indicator solution, pour the calcium chloride and baking soda mixture into the second cup.
Expected results
Calcium chloride and baking soda
Bubbles will be produced, but they will take longer to form and the amount or volume of bubbles produced will be smaller than in the reaction between citric acid and baking soda. The solution will first turn reddish-pink and then pink and the resulting mixture will be cloudier. The temperature will increase.
Ask students:
- What changes did you notice in each chemical reaction? Did the temperature increase, decrease or stay the same during the reactions?
Remind students that the gas produced in each reaction is a new substance formed as a result of a chemical reaction between the two substances. Tell students that a change in temperature is also evidence that a chemical reaction occurred. Also, each pair of chemicals changed the indicator a different color, which is evidence that different reactions occurred.
Explain
3. Show an animation to help explain that different substances react in characteristic ways.
Show the Animation Different Substances have Different Reactions.
Explain that different substances have unique or characteristic properties. One set of properties of a substance is the way it reacts with another substance in a chemical reaction.
In this activity, you mixed baking soda with citric acid in an indicator solution. You also mixed baking soda with calcium chloride in universal indicator solution. The reactions you observed between these two sets of substances are different because the particles (atoms and molecules) are different in citric acid and calcium chloride, and therefore have different chemical reactions when combined with baking soda.
Extend
4. Do a demonstration to show the chemical reaction of Alka-Seltzer with universal indicator solution.
Materials
- Clear plastic cup or graduated cylinder
- Indicator solution
- Effervescent tablet like Alka-Seltzer
Note: This demonstration is similar to the demonstration in the EXTEND in Lesson 3.2. This time, instead of putting the effervescent tablet in water with a little detergent, you will put it in universal indicator solution.

Procedure
- Show students an effervescent tablet, like Alka-Seltzer. Explain that the tablet is mostly baking soda, but it also contains both citric acid and aspirin. Aspirin, like citric acid, is an acid.
- Add 50 mL of universal indicator solution to a clear plastic cup.
Ask students:
- What do you expect to see when we put the tablet in the universal indicator solution?
Since Alka-Seltzer contains both citric acid and baking soda, it should bubble and the indicator color should change in a similar way to what we observed when we combined citric acid and baking soda in a universal indicator solution.
- Hold up the cup to display the indicator color and carefully add the Alka-Seltzer tablet to the solution.
Expected results
Bubbles are produced and the color of the solution changes, first to a reddish-color and then to yellow or yellow green.
Discuss with students that these observations make sense based on the results obtained in the experiment and observations when they added citric acid and baking soda to universal indicator.





