Lesson Overview for Teachers
View the video below to see what you and your students will do in this lesson.
Objective
Students will be able to make measurements showing that whether the process is a change of state, dissolving, or a chemical reaction, the total mass of the substances does not change.
Note: In the demonstrations and activities in this lesson, substances will be weighed before and after various processes have occurred – either melting, dissolving, or a chemical reaction. The basic principle students should observe and conclude is that mass is conserved in these processes, so the mass should not change. Students may observe slight variations of plus or minus 0.1 grams, depending on the sensitivity of the balance or whether the mass is actually somewhere between two values. If there is a minor change in mass, explain to students that small differences may be caused by a slight lack of precision in the scale readout, or by errors in the weighing methods, but that the overall results suggest that mass is conserved in all of these processes.
Key Concepts
- When a substance changes state, the mass of the substance does not change.
- When a substance dissolves in a liquid, the total mass of the substance and the liquid it dissolves in does not change.
- When substances react to form new substances as products, the mass of the products is the same as the mass of the reactants.
NGSS Alignment
- NGSS 5-PS1-2: Measure and graph quantities to provide evidence that regardless of the type of change that occurs when heating, cooling, or mixing substances, the total weight of matter is conserved.
Note: In this lesson, students measure and observe that mass is conserved during the processes of melting, dissolving, and chemical change. The students will not make a graph.
Summary
- Students check to see whether the mass of ice and water in a cup changes as the ice melts.
- Students also test whether the combined mass of sugar and water changes after sugar is dissolved in the water.
- As a demonstration, students will observe that a precipitate forms in a reaction between solutions of magnesium sulfate and sodium carbonate, and that the mass of the products is the same as the mass of the reactants.
Evaluation
Download the student activity sheet (PDF) and distribute one per student when specified in the activity. The activity sheet will serve as the Evaluate component of the 5-E lesson plan.
Safety
Make sure you and your students wear properly fitting safety goggles. Sodium carbonate may cause skin and serious eye irritation. Follow all safety precautions regarding the use, storage, and disposal of sodium carbonate.
Clean-up and Disposal
Remind students to wash their hands after completing the activity. All common household or classroom materials can be saved or disposed of in the usual manner.
Materials
Materials needed for each group
- 1 Clear plastic cup
- Water
- 1 Teaspoon of sugar
Materials for the ENGAGE demonstration
- 1 Clear plastic cup
- Water
- 1 Ice cube
- Scale
Materials for the EXTEND demonstration
- 2 Clear plastic cups
- Sodium carbonate
- Magnesium sulfate (Epsom salt)
- Water
- Graduated cylinder
- Teaspoon
Engage
1. Do a demonstration to show that melting ice in water does not cause the mass of the combined water and ice to change.
Question to investigate: Will the combined mass of water and ice stay the same as the ice in the cup melts?

Procedure:
- Pour water into a clear plastic cup so that it is about 1/3-full.
- Add 1 piece of ice.
Ask students:
- If we weigh this cup with the water and ice, do you think the combined mass will change as the ice melts?
No. - Why or why not?
Because the ice is just melting. It is still the same amount of water, but it’s just changing from a solid to a liquid. It should have the same mass.
Note: It is possible that some water may evaporate from the cup as the ice melts, causing the contents of the cup to weigh a little less at the end of the process. On the other hand, any water condensing on the outside of the cup could make it weigh a little more. Neither of these factors is likely to contribute much to the combined mass measurements, since very little water will evaporate or condense in the time it takes for the ice to melt.

- Weigh the cup, water, and ice. Record the combined mass on the activity sheet.
While the ice melts, have students conduct the experiment below. When they are done with that experiment and the ice has melted, show students the mass of the water and melted ice.
Expected results
The mass should be the same.
Give each student an Activity Sheet (PDF).
Students will record their observations and answer questions about the activity on the activity sheet.
Explore
2. Have students weigh water and sugar before and after the sugar dissolves.
Question to investigate: Will the combined mass of sugar and water be the same after the sugar dissolves in the water?
Ask students:
- If you weigh a cup of water and a teaspoon of sugar and then dissolve the sugar in the water, do you think the mass will change?
No.
- Why or why not?
Because the same amount of sugar is still there. The solid sugar crystals break apart in water as the sugar dissolves, but the individual sugar particles or molecules are still present and do not change as a result of dissolving in the water. The combined mass of the sugar and water shouldn’t change.

Materials for each group:
- 1 Clear plastic cup
- Water
- 1 Teaspoon of sugar
Procedure
- Add water to the cup until it is about 1/4-full.
- Add 1 teaspoon of sugar to the water.
- Weigh the cup with the water and sugar and record the mass.
Note: Evaporating water could make the water and sugar weigh a little less. This will probably not be a factor since very little water will evaporate in the time it takes for the sugar to dissolve.

- Carefully swirl the cup to help the sugar dissolve.
- When the sugar is dissolved, place the cup back on the scale to measure the mass.
Expected results
The combined mass does not change.
Ask students:
- Did the mass change?
No.
3. Do a demonstration to see if the mass changes during a chemical reaction.
Question to investigate: Will the mass change when reactants combine to form products in a chemical reaction?
Materials for the demonstration
- 2 Clear plastic cups
- Sodium carbonate
- Magnesium sulfate (Epsom salt)
- Water
- Graduated cylinder
- Teaspoon

Procedure
- In a clear plastic cup add 50 mL of water and 1 teaspoon of Epsom salt (magnesium sulfate). Gently swirl the cup so that the Epsom salt dissolves.
- Measure the combined mass of the cup with the Epsom salt solution in it. Tell students the mass and have them record it.
- To another cup, add 50 mL of water and add 1 teaspoon of sodium carbonate. Gently swirl the cup until the sodium carbonate dissolves.
- Measure the combined mass of the cup with the sodium carbonate solution in it. Tell students the mass and have them record it.
- Have students add the two masses together and record and announce the sum.
- Hold the cups up so students can see them and then slowly and carefully add the sodium carbonate solution to the Epsom salt solution.

Expected results
A white solid will form. At first the solid may appear or look like clouds of white particles floating in the liquid, but the particles should eventually settle out to form a solid precipitate at the bottom of the cup.
Tell students that a chemical reaction took place and that a new substance, a solid, was formed.
Ask students:
- Do you think the total mass of the two cups, the combined solutions, with the white solid will be more, less or the same as it was before the reaction took place?
Same.

- Place both cups on the scale to measure the total mass.
Expected results
The total mass should be the same as the sum of the individual masses recorded before the contents of the cups were combined and the reaction took place.
Explain that the reactants have been transformed into a new substance, but that all the individual atoms making up the reactants are still present in the products. That’s why the mass stays the same.
Explain
4. Show an animation to help explain why mass is conserved in melting, dissolving, and in a chemical change.

Show the animation Conservation of Mass in Physical and Chemical Changes.
Explain that whether a process involves melting, dissolving, or a chemical reaction, all the atoms that were there before the process takes place are still there after any changes have occurred, so the overall mass stays the same.
Extend
5. Show an animation to help explain why mass is conserved when water is frozen.
Remind students that they observed that the overall mass of water and ice stayed the same as the ice melted.
Ask students:
- When water freezes to form ice, it takes up more room in the container, but does its mass change?
Even though the volume of water changes as it becomes ice, the mass of the water should remain the same before and after it turns into ice.
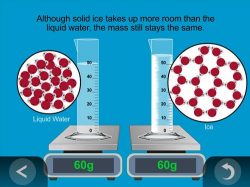
Show the animation Mass is Conserved in Freezing.
Explain that even though water takes up more room when frozen, the same number of water molecules are still there so the mass stays the same.






