Lesson Overview for Teachers
View the video below to see what you and your students will do in this lesson.
Objective
Students will design, test, modify, and optimize a device that uses a chemical reaction to produce enough gas to inflate a bag to make a cell phone float.
Key Concepts
- The goal of engineering is to design an object or process to solve a problem.
- To design a solution to a problem, engineers need to define the features that will make the object or process successful (criteria) and those that may interfere with the success (constraints).
- Engineering involves designing and testing a model or prototype and then modifying, improving, and optimizing the prototype based on the results of the testing.
- Designing a device that can make a cell phone float by using a chemical reaction requires testing, measuring, and refining the quantities of substances needed, and then modifying the materials to obtain an optimal design.
NGSS Alignment
- NGSS Standard: 3-5-ETS1-1
Define a simple design problem reflecting a need or a want that includes specified criteria for criteria and constraints on materials, time, or cost. - NGSS Standard: 3-5-ETS1-2
Generate and compare multiple possible solutions to a problem based on how well each is likely to meet the criteria and constraints of the problem. - NGSS Standard: 3-5-ETS1-3
Plan and carry out fair tests in which variables are controlled and failure points are considered to identify aspects of a model or prototype that can be improved.
Summary
This lesson begins with a design challenge: to invent a small device that uses a chemical reaction to prevent a cell phone from sinking if the phone accidentally falls into water. Rather than using a 5-E format, the lesson is organized according to the steps of the engineering design process:
Define the Problem
- Identify the Need
The lesson begins with a story about a cell phone that accidentally ends up in a lake. Rather than sinking and the phone being lost, could the phone be fitted or equipped with some type of device that would cause the phone float to the surface so it could be recovered?
- Consider Criteria and Constraints
As a class, students identify the features the device should have to be successful (criteria), as well as the factors that might limit or impede the development of a successful design (constraints).
Develop Possible Solutions
- Conduct a Test on Two Different Acids
Students prepare two solutions, with citric acid and cream of tartar, and test the two solutions with baking soda to see which produces a greater amount of gas.
- Make a Prototype
After students decide that citric acid is the better acid to use, they will combine water, citric acid, and baking soda in a small, zip-closing plastic snack bag to produce and trap carbon dioxide gas in the bag. Students test the inflated bag as a possible flotation device for a model cell phone made of clay.
Optimize the Design
- Improve your Prototype
Students will test smaller amounts of citric acid and baking soda to determine the optimum amounts that will produce enough gas to float the cell phone. Using smaller amounts of materials can reduce the overall cost, weight, and size of the flotation device and contribute to a better design. Students also look at videos of an actual flotation device and discuss and draw how some features from this product might be incorporated into a cell phone flotation device. As an optional extension, you may choose to have students continue designing their cell phone rescue device. Have students draw the device and include captions to describe the special features of their device.
Evaluation
Download the student activity sheet (PDF) and distribute one per student when specified in the activity. The activity sheet is meant as a formative assessment of student progress and understanding.
Safety
Make sure you and your students wear properly fitting safety goggles. Citric acid is an eye irritant. Read and follow all safety warnings on the label.
Clean-up and Disposal
Remind students to wash their hands after completing the activity. At the end of the lesson, have students pour their used solutions into a waste container. Dispose of this waste down the drain or according to local regulations. Any leftover citric acid or baking soda can also be rinsed down the drain with plenty of water.
All other common household or classroom materials can be saved or disposed of in the usual manner.
Materials
Materials needed for each group
- Goggles
- Citric acid
- Cream of tartar
- Baking soda
- Water
- 2 Small clear plastic cups
- Liquid dish detergent
- Dropper
- Graduated cylinder
- Measuring spoons (⅛ tsp, ¼ tsp, and ½ tsp)
- Snack size, zip-closing plastic bag
Teacher Preparation
- Label four small cups for each group: Detergent, Citric Acid, Cream of Tartar, and Baking Soda
- Make a detergent solution by adding 1 teaspoon of liquid dish detergent to 2 tablespoons of water. Divide this detergent solution equally among the labeled detergent cups to give each group one cup.
- Place 2 teaspoons of citric acid in its labeled cup for each group.
- Place 2 teaspoons of cream of tartar in its labeled cup for each group.
- Place 2 teaspoons of baking soda in its labeled cup for each group.
Before class, make or have student helpers prepare a clay cell phone for each group. The dimensions are: 12 cm long x 6 cm wide x 1 cm thick. The density of the clay model is very similar to the actual density of a real iPhone. Each clay model phone will require about 1-¼ standard bars of modeling clay.
Give each student an Activity Sheet (PDF).
Students will record their observations and answer questions about the activity on the activity sheet.


Define the problem

1. Introduce the design challenge with a story.
Set the stage for the lesson by telling students or having them read the following story. It is also written on the accompanying student activity sheet.
Imagine that you are riding in a boat enjoying the sunshine with your friends. You tell a great joke or funny story and at the punch line you spread your arms out wide. As everyone starts to laugh, you notice your cell phone flying through the air. You look in horror as the phone hits the water and begins to sink.
Have you ever dropped anything in the water and lost it? This can happen to anyone. If you are near a pool, lake, river, or ocean your cell phone could be in danger. Maybe there’s a way to protect your phone and prevent it from sinking into the watery depths.
Tell students that they will try to design a new invention or device that uses a chemical reaction to rescue a sinking cell phone.
Note: Explain to students that they will not be able to fully develop the entire device from start to finish, but they will work on the first important part of the design: finding a chemical reaction that uses a small amount of ingredients that can produce enough gas to make a cell phone float.
Ask students:
- What chemical reaction have we done so far that might be used in some way as part of a device to allow a cell phone to float if it hits the water?
Students may suggest a reaction that produces a gas, such as the reaction between vinegar and baking soda.
Tell students that they will experiment with mixtures of water, baking soda, and two different acids to identify a mixture that will produce enough gas to inflate a small plastic bag as part of a cell phone flotation device.
2. Introduce the term “engineer” and what an engineer does.
Young students can be introduced to the role of science in engineering and the engineering design process. Many students may think of “engineers” as people who operate trains. You can tell students there are many different types of engineers. Many engineers use science and technology to build devices that people need for various uses. Give students some examples of engineers and engineering:
People who plan and design roads, bridges, and tunnels are engineers. People who design ways to generate and send electricity to any place that needs it are engineers. People who design new materials, machines, and products are engineers.
Explain that an engineer is a person who uses science, math, and technology to design a process or a device to help solve problems. Tell students that in this lesson, they will be the engineers who design a cell phone flotation device.
3. Discuss the criteria and constraints for a successful design.
Explain to students that the features the device must have to be successful are called the criteria.
Ask students:
- If you think about a cell phone flotation device, what basic features do you think the device needs to have?
- It should be small and lightweight,
- Use small amounts of chemicals,
- Inflate quickly and produce enough gas to make the phone float, and
- Stay inflated.
Explain to students that possible problems that might prevent the design from successfully meeting all the criteria are called constraints.
Ask students
- What are some factors that might prevent the device from being successful?
- The chemicals might not produce enough gas to make the cell phone float.
- The device might need a large amount of chemicals or a large bag to make it work, which could make the device too expensive and too big.
- The bag might tear or break the seal, causing either the liquid or the gas to leak.
Develop possible solutions
4. Compare the amount of gas produced when citric acid or cream of tartar solution reacts with baking soda.
Tell students that part of designing a product is to test different ingredients to see what works best. Explain that they will compare two different acids to see which one produces the most gas when it reacts with baking soda. Explain that the acids they will use are powders that are used in food. Students will add water to these powders to make acid solutions. In order to visually compare how much gas is produced, they will add one drop of detergent solution to each acid solution. The detergent will cause gas produced by the reaction to create foam. Students will compare the amounts of foam produced to determine which acid solution produces more gas when it reacts with baking soda.
Question to investigate: Which acid produces more gas when it reacts with baking soda?
Materials for each group
- Goggles
- Citric acid
- Cream of tartar
- Baking soda
- Detergent solution
- Water
- 2 Small clear plastic cups
- 2 Wide clear plastic cups
- Graduated cylinder (50- or 100-mL)
- Dropper for detergent solution
Procedure
- Pour 10 mL of water into each of two small clear plastic cups.
- Add ¼ teaspoon of citric acid to one cup.
- Add ¼ teaspoon of cream of tartar to the other cup.
- Carefully swirl each cup until the powder dissolves as much as possible. (The cream of tartar will not dissolve completely and the resulting solution will look milky white.)
- Add 1 drop of detergent solution to each cup and gently swirl to mix.
- Add ¼ teaspoon of baking soda to the graduated cylinder and stand the cylinder upright in a wide, clear plastic cup.
- Pour the cream of tartar solution into the graduated cylinder with the baking soda. Observe and record the amount (level) of foam in the graduated cylinder.
Expected results
The foam will rise to about the top of a 50-mL graduated cylinder.
- Rinse out the graduated cylinder, dry it with a paper towel, and add ¼ teaspoon of baking soda to the cylinder. Stand the graduated cylinder upright in a wide, clear plastic cup.
- Carefully pour the citric acid solution into the graduated cylinder containing the baking soda. Observe and record the amount (level) of foam in the graduated cylinder.
Expected results
The foam will rise faster and higher with the citric acid solution than it did with the cream of tartar solution. The foam may overflow a 50-mL size graduated cylinder.
Ask students
- Which acid produces more gas when it reacts with baking soda?
Citric acid produces more gas when it reacts with baking soda.
- Which acid should we use in our cell phone rescuing device? Why?
We should use citric acid because it reacts faster than cream of tartar and produces more gas.
Remind students that citric acid and baking soda are called the reactants in this chemical reaction. Reactants are the chemicals that are mixed together and interact during a chemical reaction. Carbon dioxide gas is one of the products of this chemical reaction. Products are new substances that are produced during a chemical reaction.



5. Have students mix citric acid, baking soda, and water in a plastic bag to see how much it inflates.
Remind students that 10 mL of water, ¼ teaspoon of citric acid, and ¼ teaspoon of baking soda created a lot of foam in the graduated cylinder.
Have students test these same amounts of reactants in a small, snack-size, zip-closing plastic bag to see how much the bag inflates.
Question to investigate: How full will a bag inflate when citric acid, baking soda, and water are combined in a sealed plastic bag?
Materials for each group
- Goggles
- Citric acid
- Baking soda
- Water
- Graduated cylinder
- Snack size zip-closing plastic bag
Procedure
- Open a snack-size, zip-closing plastic bag. Work with a partner to add ¼ teaspoon of citric acid to one corner of the bag.
- Add ¼ teaspoon of baking soda to the same corner of the bag. Using your fingers, gently knead or rub the outside of the bag to mix the powders together.
- Use your fingers to hold or close off the corner of the bag containing the powder to keep the solids separate from the rest of the bag. Have your partner carefully add 10 mL of water to the other corner of the bag. Try to prevent the water from touching the powders in the other corner.
- Get as much air out of the bag as you can and seal the bag securely. Let go of the twisted area and tilt the bag back and forth so that the water and the powders mix.
- Lay the bag on a table and see how much gas is produced and how much the bag expands.
Expected results
Gas should be produced and fill up the bag almost all the way.


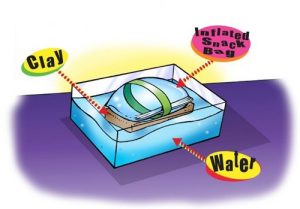
6. Use the inflated bag to see if it can make a clay cell phone float.
Materials for each group
- Clay model of a cell phone
- 1 Piece of tape (about 25–30 cm)
- Water
- Bucket or large plastic container
Procedure
- Attach the bag to the clay model by wrapping a piece of tape around the bag and clay.
- Place the taped bag and clay in a bucket or large container of water.
Expected results
The bag floats with the clay phone just under the surface of the water.
Optimize the Design
7. Test smaller amounts of ingredients to see if they will still make the cell phone float.
Explain to students that cost is an important factor when designing and developing a product. If the amount of ingredients can be reduced and still make the cell phone float, then it is good to use a smaller amount. Using fewer materials will reduce the cost of the
Question to investigate: Can smaller amounts of ingredients still produce enough gas to inflate the plastic bag and make the cell phone float?
Materials for each group
- Goggles
- Citric acid
- Baking soda
- Water
- Graduated cylinder
- Snack size zip-closing plastic bag
- 1/8 teaspoon
- Clay model of a cell phone
- 1 Piece of tape (about 25–30 cm)
- Water
- Large bowl or bucket
Procedure
- Open a small, snack-size, zip-closing plastic bag. Work with a partner to add 1/8 teaspoon of citric acid to one corner of the bag.
- Add 1/8 teaspoon of baking soda to the same corner of the bag. Using your fingers, gently knead or rub the outside of the bag to mix the powders together.
- Use your fingers to hold or close off the corner of the bag containing the powder to keep the solids separate from the rest of the bag. Have your partner carefully add 10 mL of water to the other corner of the bag. Try to be sure that the water does not yet touch the powders in the other corner.
- Get as much air out of the bag as you can and seal the bag securely. Let go of the corner and tilt the bag back and forth so that the water and the powders mix.
- Lay the bag on a table and see how much gas is produced and how much the bag expands.
Expected results
Gas should be produced and partially inflate the bag.
8. Use the partially inflated bag to see if it still allows the clay model cell phone to float when placed in water.
Procedure
- Attach the bag to the clay model by wrapping a piece of tape around the bag and clay.
- Place the taped bag and clay in a large bowl of water.
Expected results
The bag floats with the clay phone just under the surface of the water.
9. Discuss other design features that would make the self-inflating bag work as part of a cell phone flotation device.
Explain to students that the inflatable bag is a good first step in designing a cell phone flotation device. But the bag alone would not be enough to rescue a phone. Other inventions would need to be combined with the self-inflating bag to create a possible rescue device.
Explain to students that there would need to be a way to keep the water and the powder mixture separated until the phone hits the water. There would also need to be some kind of sensor or mechanism to cause the powders and the water to mix when the cell phone hits the water.

Show the video Water Buoy.
Explain to students that such a device, called the Water Buoy, inflates when it sinks in water to rescue items that have fallen overboard.
This device uses a balloon and a small canister of compressed carbon dioxide gas. These fit into a small container that can be attached to a set of keys or other object. If the item falls in the water, the plug sealing the canister dissolves, releasing the gas, which inflates the balloon. Then the object floats to the surface of the water. The balloon is bright orange and lights up to make it easy to spot and retrieve.
Optional
10. Have student groups discuss, draw, and explain how the flotation device might be activated when it hits the water.
Discuss with students the idea of a moisture or pressure sensor that could be used to activate the chemical reaction when the cell phone begins to sink in water. See if students have other ideas.
Give students the opportunity to draw a possible rescue device in both normal use and rescue mode. Students can incorporate any of the ideas discussed or new ones into their own water-rescue device for phones. Students should use captions to describe the different features of their device. Once students have completed their drawings, you may choose to have them share their designs with the class.





