Lesson Overview for Teachers
View the video below to see what you and your students will do in this lesson.
Objective
Students will be able to plan and carry out an investigation to compare the solubility of different substances, and develop and explain a particle-level model to describe the process of dissolving. Students will also be able to explain that substances dissolve by different amounts because of the molecules they are made from.
Key Concepts
- For a dissolving test to be fair, the same amount of each substance being tested, and the same amount of water need to be used.
- The amount of a substance that dissolves in a certain amount of water is a characteristic property of that substance.
- The different atoms and molecules of a substance give it its characteristic solubility.
NGSS Alignment
- NGSS 5-PS1-3: Make observations and measurements to identify materials based on their properties.
Summary
In earlier grades, students observed and tested objects and materials, and saw that the items could be grouped based on their characteristic properties. In this and the following lessons in Chapter 2, students continue to develop this idea and see that the characteristic properties of a substance can be used to identify the substance.
In this lesson:
- Students are given labeled samples of salt and sugar. They are also given unknown samples marked A, B, and C. One is salt, one is sugar, and the other is alum, which looks like it could be either salt or sugar.
- Students first use a dissolving test to see how salt and sugar dissolve in water. Students then run the same dissolving test on substances A, B, and C; identify the salt and sugar; and conclude that the other substance must be something different.
- Students then see an animation to help explain that the substances are made of different atoms and molecules, so they dissolve differently.
Evaluation
Download the student activity sheet and distribute one per student when specified in the activity. The activity sheet will serve as the Evaluate component of the 5-E lesson plan.
Safety
Make sure you and your students wear properly fitting safety goggles.
Clean-up and Disposal
Remind students to wash their hands after completing the activity.
All common household or classroom materials can be saved or disposed of as you ordinarily would.
Teacher preparation
Materials
- Sugar
- Salt
- Alum
- Dirt
- Water
- 8 clear plastic cups (per student group)
- Teaspoon
- 3 tall clear plastic cups
Preparing the materials
- Label 5 cups Sugar, Salt, A, B, and C for each group.
- Place 1 teaspoon of sugar and 1 teaspoon of salt in their labeled cups.
- Place 1 teaspoon of alum in cup A, place 1 teaspoon sugar in cup B, and place 1 teaspoon salt in cup C.
Do not tell students what is in cups A, B, and C.
Materials for each group
- Sugar in labeled cup
- Salt in labeled cup
- Cup labeled A with alum
- Cup labeled B with sugar
- Cup labeled C with salt
- 3 empty clear plastic cups labeled A, B, and C
- 3 empty cups (to measure and pour water from)
- Water
- ½ teaspoon
- Graduated cylinder or teaspoon

Engage
1. Have a class discussion about how to tell the difference between salt and sugar (without tasting).
Hold up two clear cups of salt and sugar. Tell students that salt and sugar look very similar but, as students know, they are very different.
Ask students:
- Other than tasting the salt and sugar, what test could we do to see how salt and sugar are similar or different?
Students may suggest comparing how they smell or comparing how easy or hard it is to crush them. If students do not suggest a dissolving test, you can suggest it.
Tell students that they will investigate whether it is possible to tell one substance from another based on how much the substance dissolves. Tell students that they will first compare how well salt and sugar dissolve in water. Then they will use their results to figure out what the unknown substances are in cups A, B, and C. Tell students that one contains salt, one contains sugar, and the other contains either salt, sugar, or something else. Explain that the class will design a dissolving test to try to figure out what substance is in which cup.
Give each student an Activity Sheet (PDF).
Students will record their observations and answer questions about the activity on the activity sheet.
Explore
2. Help students design a dissolving test.
Have a discussion with students about the best way to design a dissolving test to see if there is a difference between salt and sugar.
Question to Investigate: Can you identify substances based on how well they dissolve in water?
Materials for each group
- Sugar in labeled cup
- Salt in labeled cup
- Cup labeled A with alum
- Cup labeled B with sugar
- Cup labeled C with salt
- 3 empty clear plastic cups labeled A, B, and C
- 3 empty cups (to measure and pour water from)
- Water
- ½ teaspoon
- Graduated cylinder or teaspoon
Ask students:
- If we want to compare the dissolving (solubility) of sugar and salt, should we use the same amount of sugar and salt?
Yes - Should we put them in the same amount of water to dissolve them?
Yes - Should the water be at the same temperature?
Yes
Procedure
- Place ½ teaspoon of salt and ½ teaspoon of sugar into their labeled cups.
Note: A solubility test is normally measured by the mass of a substance that dissolves in a given volume of water. Using volume (1/2 teaspoon) for the salt and sugar instead of mass is probably acceptable for this age group for showing that different substances have different solubilities. In middle school, students can weigh the solutes for a solubility test that uses equal masses.

- Add 10 milliliters (2 teaspoons) of water to two separate cups.
- At the same time, pour the water into the sugar and salt cups.
- Gently swirl the cups to see whether sugar or salt dissolves the most.
Expected results
The sugar will dissolve completely but some salt will remain undissolved.
Ask students:
- What did you observe?
The sugar dissolved completely and faster than the salt. The salt dissolved much more slowly than the sugar and did not dissolve completely, but almost.
3. Help students design and conduct a dissolving test on the substances in cups A, B, and C.
Discuss with students how they should test the substances in cups A, B, and C. Students should understand that they need to test them the same way they tested the sugar and salt so they can compare them to what they saw before.

- Place ½ teaspoon from cup A into empty cup A. Place ½ teaspoon from cup B into empty cup B. Place ½ teaspoon from cup C into empty cup C.
- Add 10 milliliters of water (2 teaspoons) to 3 empty cups.
- At the same time, you and a partner pour the water into cups A, B, and C and gently swirl the cups to see which dissolves most similarly to sugar and salt. Also note whether any of the powders could be something other than sugar or salt based on how it dissolves.
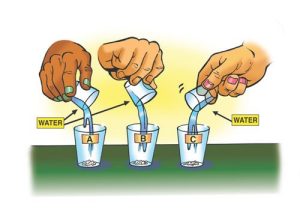
Expected results
The substance in cup B dissolved completely and the fastest. It was like the sugar. The substance in cup C dissolved more slowly than the sugar and did not dissolve completely. It was like the salt. The substance in cup A didn’t seem to dissolve much. It dissolved even slower than the salt and there was a lot that didn’t dissolve. It was not like sugar or salt.
Ask students:
- Which cup do you think had the sugar?
B - Which cup do you think had the salt?
C - Which cup do you think had a different substance?
A
Let students know that the other substance is called alum.
Explain
4. Explain that the different atoms and molecules of a substance make it dissolve in a characteristic way.
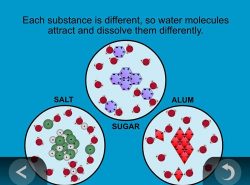
Show the animation Dissolving Different Substances.
Note: The animation uses two types of models. For the salt, the model shows the sodium and chloride ions as individual spheres with their respective charges. For the sugar and alum, no individual atoms are represented. The structures of these molecules are complicated and made up of many atoms bonded together in complex ways. Therefore, simple shapes of hexagons and diamonds with positive and negative charges are used to represent the molecules of these substances.
Explain that since the atoms and molecules that make up sugar, salt, and alum are different, and have different charges, each substance has a different structure. They also, as a result, interact differently with water molecules. Because each substance is made from different atoms in different arrangements, they dissolve differently.
Extend
5. As a demonstration, have students add salt, sugar, and alum to dirty water to show that alum cleans water more effectively than salt or sugar.
Alum has an interesting property that is different from many other substances. One way to tell the difference between salt, sugar, and alum is to do a water cleaning test.
Materials for the demonstration
- 3 tall clear plastic cups
- Masking tape
- Water
- Dirt
- Salt
- Sugar
- Alum
- ½ teaspoon
- Plastic spoon for stirring
Preparing the materials
- Use masking tape and a marker to label the three clear plastic cups Salt, Sugar, and Alum.
- Add water to the three cups until they are about ¾-full.
- Add about 1 teaspoon of dirt to each cup and stir until the water looks dirty in all the cups.

Demonstration
Procedure
- Have three students add ½ teaspoon of salt to its labeled cup, ½ teaspoon of sugar to its cup, and ½ teaspoon of alum to its cup.
- Stir the mixture very well in all three cups.
- Let them sit undisturbed and check them in about 15 – 30 minutes.
Expected results
The water in the container with the alum should look clearer than the water with the salt or sugar. It should only take 10 - 15 minutes to see a noticeable difference.
This test shows that alum has a characteristic property of being able to make dirty water clearer. It is also another way of telling that the alum is different from salt and sugar even though it looks similar to both.





