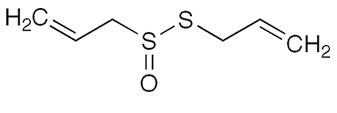
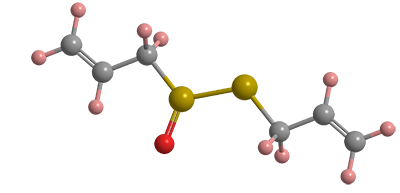
When garlic is crushed or otherwise damaged, allicin is released, giving off its recognizable odor. Allicin does not naturally occur in garlic but rather is the result of the amino acid alliin combining with the enzyme alliinase. Although research has shown that it is not bioavailable, studies confirm that allicin inhibits the bacteria responsible for gastric ulcers and lowers the risk of stomach cancer.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

