What molecule am I?
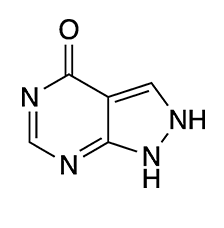
Allopurinol is best known as the “gout medicine”. By inhibiting the xanthine oxidase enzyme, it decreases high concentrations of uric acid in the blood. It is used to prevent or treat gout, uric acid–based kidney stones, and (formerly) chemotherapiy-induced uric acid formation in the blood.
Roland K. Robins at New Mexico Highlands University (Las Vegas) and P. Schmidt and J. Druey at CIBA (Basel, Switzerland) independently reported allopurinol syntheses in 1956. At the time it was known that purine derivatives acted as antagonists of some biological processes. Robins believed that derivatives of purine’s isomer pyrazolo [3,4-d]pyrimidine also might be biologically active. He synthesized 15 compounds, including allopurinol. He was looking specifically for new antitumor agents.
Schmidt and Druey also prepared allopurinol and other pyrazolo [3,4-d]pyrimidines in search of new pharmaceuticals for CIBA. In subsequent years, numerous investigators established allopurinol’s medicinal properties. The Food and Drug Administration approved it for use in the United States in 1966.
Allopurinol also has theoretical interest. In 1996, Begoña Hernández, Francisco J. Luque, and Modesto Orozco at the University of Barcelona used computational methods to evaluate the relative stabilities of tautomers of allopurinol and its isomer hypoxanthine.
To avoid having to use a large number of expensive calculations (remember, this was 1996), the authors first used a low-level method to identify the seven most stable forms of both isomers. They then used ab initio quantum mechanical methods to determine the most stable tautomer of each molecule in the gas phase and in aqueous solution.
The tautomer shown here for allopurinol and the equivalent structure of hypoxanthine were the most stable in both media. But the relative stabilities of the other tautomers differed between the two phases. The authors believed that their work would help explain the biological activites of these molecules.
Allopurinol fast facts
| CAS Reg. No. | 315-30-0 |
| Molar mass | 136.11 g/mol |
| Formula | C5H4N4O |
| Appearance | White crystals or powder |
| Melting point | >350 °C |
| Water solubility | 480 mg/L |
Chemistry Meets the Eclipse
While you observed today’s total eclipse of the sun, atmospheric scientists were studying the effect of photochemistry on new particle formation in the Earth’s atmosphere. Much of their work will extend the 2015 work of a Finnish, Swiss, and American team in which they showed that during a partial eclipse the formation of sulfuric acid and highly oxidized nitrogen compounds decreased markedly. Stay tuned for results from today’s total eclipse!

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

