What molecule am I?
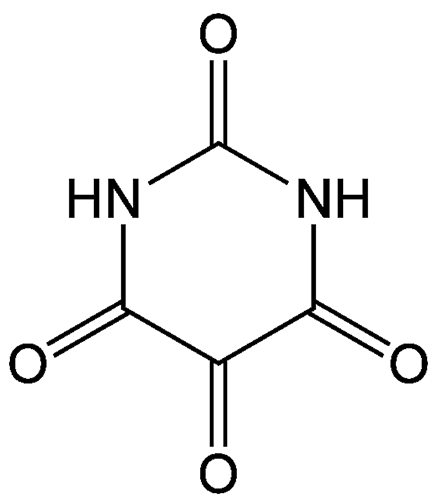
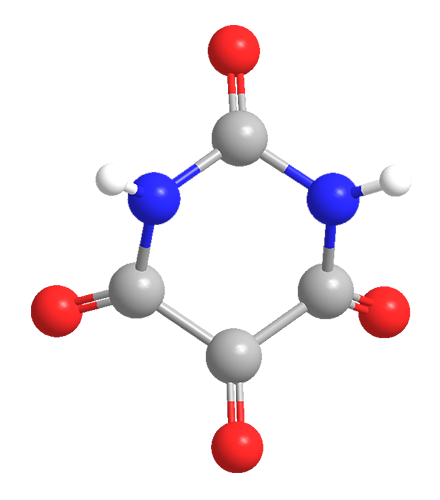
Alloxan is a thermally stable white solid with an unusual structure. Its formal name is 2,4,5,6(1H,3H)-pyrimidinetetrone, indicating that it has a pyrimidine ring structure with four carbonyl groups. It is freely soluble in water, forming a slightly acidic solution. The solid begins to decompose at 256 ºC.
In 1818, Italian chemist Luigi V. Brugnatelli was the first to isolate alloxan; he synthesized it via nitric acid oxidative degradation of uric acid. Shortly after Friedrich Wöhler found that urea can be made from inorganic materials in 1828, he and Justus von Liebig discovered alloxan in human excretions, showing that it also can be biosynthesized. Currently, alloxan is prepared from barbituric acid or alloxantin; the article of commerce is the monohydrate.
Alloxan is sometimes used in dye manufacture, but its main (and most notorious) use is to induce diabetes in laboratory rodents. Alloxan’s structure mimics that of glucose, which allows it to be absorbed by the pancreas. Once inside the organ, it destroys insulin-producing β-cells and produces a disease similar to type 1 diabetes in humans. Fortunately, alloxan is not taken up by the human pancreas, but it has shown liver and kidney toxicity.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

