What molecules are we?
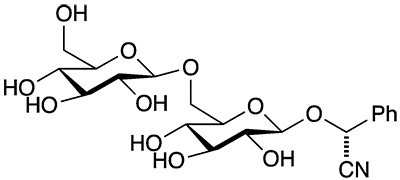
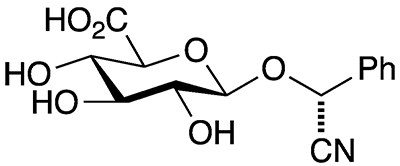
Amygdalin (on the left) and its companion molecule laetrile (right) burst into the headlines in the 1950s as purported cancer cures. As scientific research soon determined, they are not effective for treating cancer—they are potent poisons.
Amygdalin occurs in many plants, notably in seeds of fruits in the Rosaceae family such as bitter almonds, apricots, and plums. Laetrile is a derivative of amygdalin formed by the hydrolytic removal of one glycoside group from the parent compound. The literature is a bit confusing because the names and laetrile sometimes are conflated. Both molecules are also erroneously termed vitamin B17.
Chemists Walter Norman Haworth and Birkett Wylam at Durham University (Newcastle upon Tyne, UK) elucidated the structure of amygdalin and synthesized it in 1923. Haworth was awarded the 1937 Nobel Prize in Chemistry for 1938.
From the 1950s through the 1970s, both compounds, under the name laetrile, were heavily promoted as a cure for many types of cancer. Rogue physicians and others established clinics in Mexico to which desperate patients flocked to receive the “cure” and other ineffective treatments. Science, however, prevailed: Laetrile was never approved by the US Food and Drug Administration or the European Commission.
The nitrile groups in amygdalin and laetrile are labile and easily removed as the cyanide ion by β-glucosidase enzymes in the human body. Cyanide, of course, is highly toxic; it is surprising that the compounds’ oral toxicity is given the mild hazard statement “harmful if swallowed”.
In 1981, Irving J. Lerner at the University of Minnesota Medical School (Minneapolis) summarized the laetrile hoax thus: "All prior forms of cancer quackery . . . pale in comparison with the laetrile crusade, the slickest, most sophisticated, and certainly the most remunerative cancer quack promotion in medical history."
Amygdalin and laetrile* hazard information
| GHS classification**: acute toxicity, oral, category 4 | |
| H302—Harmful if swallowed | |
*No safety data sheet for laetrile is available. Hazards are assumed to be the same as for amygdalin.
**Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Amygdalin fast facts
| CAS Reg. No. | 29883-15-6 |
| Empirical formula | C20H27NO11 |
| Molar mass | 457.43 g/mol |
| Appearance | White to beige powder |
| Melting point | 223–226 ºC |
| Water solubility | 50 g/L |
Laetrile fast facts
| CAS Reg. No. | 1332-94-1 |
| Empirical formula | C14H15NO7 |
| Molar mass | 309.27 g/mol |
| Appearance | White powder |
| Melting point | 214–216 ºC |
| Water solubility | 83 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

