What molecule am I?
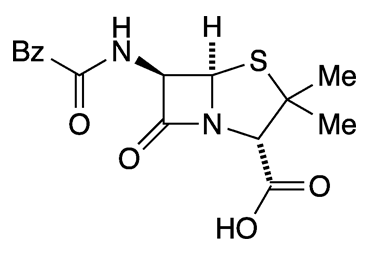
The “wonder drug” penicillin is actually a group of drugs that have different functional groups on one of the side chains on the bicyclic core. In 1928, Scottish biologist Alexander Fleming isolated the first specific form of penicillin from Penicillium fungi; the compound is known variously as benzylpenicillin, penicillin G, or benzylpenicillinic acid. For this achievement, Fleming shared the 1945 Nobel Prize in Physiology or Medicine.
The free acid benzylpenicillin is only sparingly soluble in water; but even worse, it is inactivated by water. Thus, the articles of commerce are its sodium and potassium salts, which are much more water-soluble and stable in solution.
By the beginning of World War II, penicillin had been developed into antibacterial drugs that could be mass-produced. At the time of the Normandy invasion in 1944, more than 2 million doses had been made. Penicillin may have saved as many as 100,000 lives during the war.
Much more recently, it was discovered that some soil bacteria readily consume penicillin and other β-lactam antibiotics. Biologist Gautam Dantas and his team at Washington University (St. Louis) hypothesized that bacteria engineered to maximize β-lactam consumption capacity could be used to remediate antibiotic-contaminated soils.
The researchers created a strain of Escherichia coli that uses penicillin as its only carbon source. The downside, however, is that other organisms might also acquire the degradation genes and become resistant to penicillin.
Benzylpenicillin hazard information
| LCSS classification*: sensitisation, skin, category 1 | |
| H317—May cause an allergic skin reaction | |
| LCSS classification: sensitisation, respiratory, category 1 | |
| H334—May cause allergy or asthma symptoms or breathing difficulties if inhaled | |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Benzylpenicillin
fast facts
| CAS Reg. No. | 61-33-6 |
| Molar mass | 334.39 g/mol |
| Empirical formula | C16H18N2O4S |
| Appearance | White crystals or powder |
| Melting point | 82–83 ºC |
| Water solubility | 210 mg/L |
MOTW update
Cannabidiol (CBD) was the Molecule of the Week for February 6, 2017. CBD is the term for a family of cannabinoids that are isomers of THC, the chief psychoactive component of marijuana. These compounds are not psychoactive, but they are useful for controlling pain and other medical conditions. Up to now, CBD had an unclear regulatory status; but it now has its first approval from the US Food and Drug Administration. On June 25, FDA approved an oral solution of CBD for treating seizures from two severe forms of epilepsy.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

