What molecule am I?
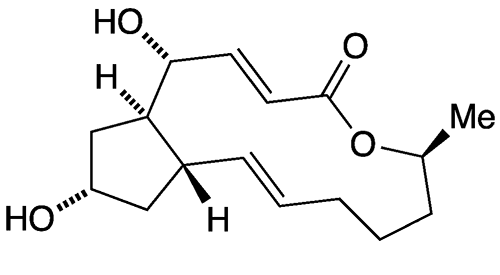
Brefeldin A1 (BFA) is a bicyclic lactone produced by fungi of the genus Penicillium. In 1958, V. I. Singleton*, N. Bohonos, and A. J. Ullstrup at Purdue University (Lafayette2, IN) isolated it from the species P. decumbens and named it decumbin. Five years later, H. P. Sigg* and co-workers at Sandoz (Basel, Switzerland) isolated it from another species, P. brefeldianum and changed its name to brefeldin A.
Sigg and his group went on to determine BFA’s biosynthesis (1968) and elucidate its structure (1971). Several total syntheses followed, including that of E. J. Corey* and Robert H. Wollenberg at Harvard University (Cambridge, MA) in 1976, who made the racemic compound. In 1990, Corey and Philip Carpino reported a simplified synthesis of the (+)-enantiomer.
Why all the interest in BFA? It was recognized early on to have antiviral properties, as in a 1968 report by Seikichi Suzuki and coauthors at the University of Tokyo and Chugai Pharmaceutical (Tokyo), who observed moderate activity in two in vitro studies. But subsequent research did not result in its development as an antiviral drug.
BFA, however, found new life as a model compound for the study of protein transport. Beginning in 1986, Yukio Ikehara and collaborators at the Fukuoka University School of Medicine, Saga Medical School, and the University of Tokyo (all in Japan) demonstrated that BFA strongly blocks protein secretion in cultures of rat hepatocytes.
Then, in a 2002 article titled “Brefeldin A: Deciphering an Enigmatic Inhibitor of Secretion”, Andreas Nebenführ, Christophe Ritzenthaler, and David G. Robinson*3 reported on their study of dozens of plants and other eukaryotes and concluded: “The molecular target for BFA appears to be the same in all eukaryotic cells, namely, a Sec7-type GEF that is necessary for activation of Arf1p.” For the biochemically uninitiated, GEF is guanine nucleotide exchange factor; Sec7 is a domain responsible for some GEF catalytic activity; and Arf1p is a hydrolase enzyme that binds to the nucleotide guanosine triphosphate.
1. SciFinder: 4H-Cyclopent[f]oxacyclotridecin-4-one, 1,6,7,8,9,11a,12,13,14,14a-decahydro-1,13-dihydroxy-6-methyl-, (1R,2E,6S,10E,11aS,13S,14aR)-.
2. Now West Lafayette.
3. Authors at the University of Tennessee (Knoxville), the Institute of Molecular Biology of Plants of the CNRS (Strasbourg, France), and the University of Heidelberg (Germany), respectively,
Brefeldin A hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 3 | H301—Toxic if swallowed | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms
Molecules from the journals
Scandium carbide1 (Sc2C) is an ionic, as opposed to covalent, carbide that formally contains a C4– anion. Its properties, including crystal structure, were first investigated in the late 1960s.
In June of this year, Scott C. Warren and co-workers at the University of North Carolina at Chapel Hill reported that Sc2C is a 2-D semiconducting electride. (An electride is a material with some of its electrons located in well-defined lattice sites rather than within its atoms, i.e., they are “free” electrons.) Scandium is the most electronegative metal yet found adjacent to an electride site. The authors’ computations showed that “higher electronegativity of the cation drives greater hybridization between metal and electride orbitals, which opens a band gap in these materials,” revealing Sc2C as the first 2-D electride semiconductor.
Lonafarnib2 is the first drug developed to treat Hutchinson–Gilford progeria syndrome, an ultrarare premature aging disease in children. The US Food and Drug Administration approved it for use in 2020; the agency describes it as a first-in-class medication.
Because the disease is so rare (≈400 children worldwide), lonafarnib is considered to be an orphan drug. Its developer, Eiger BioPharmaceuticals (San Carlos, CA), originally targeted the drug against hepatitis D, but when it found that lonafarnib could treat progeria, it needed a partner for outsourcing registration, validation, and commercialization. In 2015, Eiger hired CordenPharma International (Plankstadt, Germany), a contract development and manufacturing organization, to do the job. CordenPharma manufactures lonafarnib in Boulder, CO.
1. CAS Reg. No. 12202-81-2.
2. CAS Reg. No. 193275-84-2.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Brefeldin A fast facts
| CAS Reg. No. | 20350-15-6 |
| Empirical formula | C16H24O4 |
| Molar mass | 280.36 g/mol |
| Appearance | White crystals or powder |
| Melting point | 204–205 °C |
| Water solubility | Very slight |
MOTW update
Methotrexate1 was one of three Molecules of the Week for January 16, 2012. It is an “antifolate” medication used in cancer chemotherapy; but it has toxic side effects and must be closely monitored. Recently, Yaman Göksel and colleagues at the Technical Institute of Denmark (Lyngby) and other Danish institutions reported the development of a rapid, relatively inexpensive electrochemically assisted surface-enhanced Raman spectroscopy method for detecting methotrexate in human serum in a clinically relevant concentration range.
1. CAS Reg. No. 59-05-2.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

