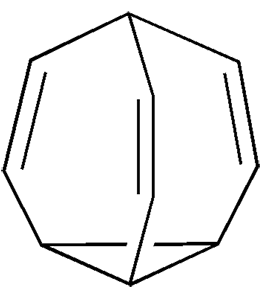
Bullvalene has a fluxional, or constantly changing, structure. Each carbon atom has an equal probability of being bonded to each of the other carbon atoms, so the proton NMR spectrum is a sharp singlet peak. Bullvalene (so named because early skeptics doubted its existence) can take on any of 1.2 million arrangements.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

