What molecule am I?
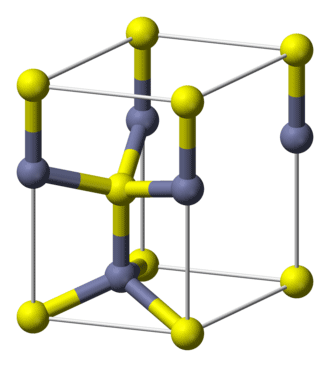
Cadmium selenide (CdSe) is a binary, primarily ionic compound with a touch of covalent character. It can take three crystalline forms, but the hexagonal wurtzite structure shown is by far the most common. The unit cell is shown in the image; the gray balls are cadmium, and the yellow balls are selenium.
In 1879, French chemist M. J. Margottet prepared CdSe by heating elemental cadmium in a current of hydrogen selenide (H2Se), then subliming the product in a hydrogen atmosphere. Since then it also has been produced by treating cadmium chloride or sulfate with H2Se. Now, in the age of nanotechnology, CdSe is made by the reaction of cadmium oxide and elemental selenium in a trialkylphosphine solvent.
CdSe is an n-type semiconductor, which makes it ideal for nanotech applications. Its nanoparticles are especially useful as a component of photocatalysts. In 2016, Philip Kalisman, Yifat Nakibli, and Lilac Amirav* at the Technion–Israel Institute of Technology (Haifa) showed that water splitting via visible-light irradiation with platinum-tipped CdSe@CdS nanorods, converts 100% of the radiated photons to hydrogen. The authors “believe that this approach . . . will enable us to tackle the remaining challenges with an eye toward overall water splitting.”
Then this year, Wenfa Xie, Hanzhuang Zhang, and colleagues at Jilin University (Changchun, China) used a spray process to make CdSe-containing quantum-dot light-emitting diodes (QLEDs) These QLEDs are 5.4 times more efficient than previously reported similar devices. The authors believe that their results “will spur the progress toward realization of high performance and mass production of all-inorganic QLEDs as a platform for QD-based full-color displays.”
We present CdSe to commemorate National Nanotechnology Day, October 9th.
Cadmium selenide fast facts
| CAS Reg. No. | 1306-24-7 |
| Molar mass | 191.37 g/mol |
| Formula | CdSe |
| Appearance | Red to black crystals |
| Melting point | 1258 ºC |
| Water solubility | Insoluble |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

