What molecule am I?
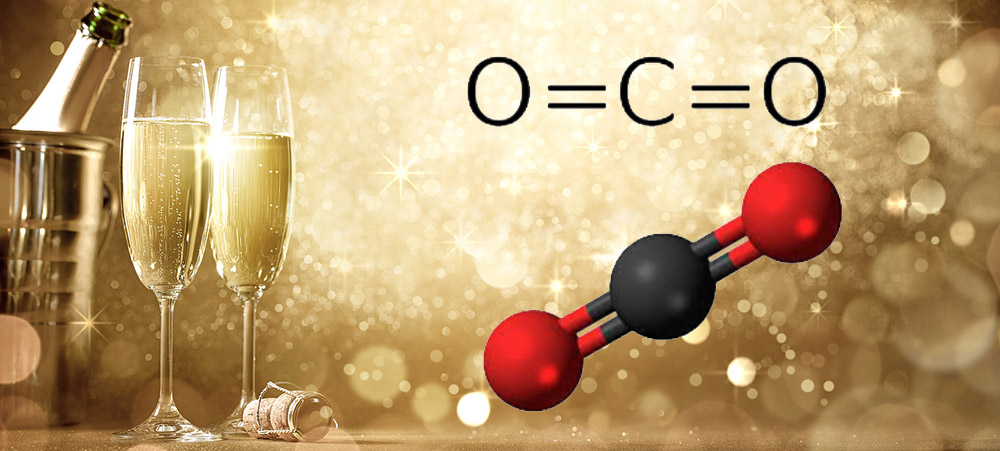
Carbon dioxide (CO2) is a long-known and thoroughly studied molecule. It has its upsides, such as its uses in carbonated beverages and urea manufacture, and its downsides, especially its dominant role in climate change. But how is it related to New Year’s Eve?
MOTW suspects that many, if not most, of its readers celebrated the incoming year with a toast of Champagne or a similar sparkling wine. If so, you may have been transfixed by the tiny CO2 bubbles emanating from your beverage. In 2014, the Web site Compound Interest published some interesting facts about CO2 in Champagne:
- A 750-mL bottle of Champagne contains ≈5 L of mostly dissolved CO2 gas.
- The CO2 gas pressure in this Champaign bottle is 5–6 atm.
- A 100-mL Champaign flute releases ≈20 million CO2 bubbles.
If this isn’t enough information for you, read a 2005 review titled “The Physics and Chemistry behind the Bubbling Properties of Champagne and Sparkling Wines” by Gérard Liger-Belair at the University of Reims Champagne–Ardenne (Reims, France).
The MOTW team wishes all our faithful readers a peaceful, healthy, and happy 2024. We are especially grateful to all of you who make our job easier by suggesting fascinating molecules.
Carbon dioxide hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Gases under pressure, liquefied gas | H280—Contains gas under pressure; may explode if heated; may displace oxygen and cause rapid suffocation | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules in the News
Caprolactam1, the Molecule of the Week for September 1, 2016, is a cyclic amide and the monomer used for making the polymer nylon 62. This strong, durable, flexible polymer has wide-ranging uses and requires a global annual caprolactam production of ≈7 million tonnes.
But what happens to used nylon 6 that needs to be recycled? Last month, Linda J. Broadbelt, Yosi Kratish, Tobin J. Marks, and colleagues at Northwestern University (Evanston, IL) described a process that decomposes nylon 6 back to its caprolactam monomer. The low-temperature, solventless process uses a lanthanide-based metallocene catalyst system to efficiently depolymerize the nylon.
Did you have a hangover from drinking too much alcohol on New Year’s Eve? If so, you may want to try a beverage that contains (R)-1,3-butanediol3 in place of ethanol. KetoneAid of Falls Church, VA, markets gin and tonic and Moscow mule cocktails that contain (R)-1,3-butanediol under the brand name “Hard Ketones”.
How does (R)-1,3-butanediol prevent hangovers? Consumed ethanol metabolizes to acetaldehyde, which causes the nasty morning-after symptoms. But (R)-1,3-butanediol metabolizes to acetoacetates—keto anions that already exist in our bodies.
1. CAS Reg. No. 105-60-2.
2. CAS Reg. No. 25038-54-4.
3, CAS Reg. No. 6290-03-5.
Molecules in the News
MOTW highlights molecules that appear in major news outlets. See this week's edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Carbon dioxide fast facts
| CAS Reg. No. | 124-38-9 |
| SciFinder nomenclature | Carbon dioxide |
| Empirical formula | CO2 |
| Molar mass | 44.01 g/mol |
| Appearance | Colorless gas |
| Sublimation pointa | –78.5 °C |
| Water solubility | 1.69 g/L (20 °C, 1 atm) |
a. Solid CO2 (“dry ice”) at ambient pressure sublimes (or “boils”) at a lower temperature than it melts. At pressures >5.1 atm (its “triple point”), it melts at –56.6 °C.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

