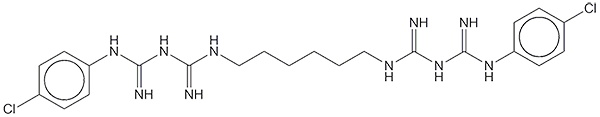
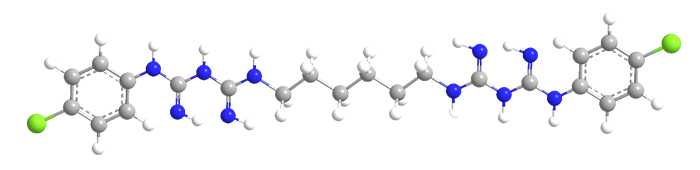
Chlorhexidine consists of a straight-chain hexane with p-chlorophenylbiguanide groups attached to both ends. It is used mostly as its salts, such as the dihydrochloride or diacetate, and not the free base. F. L. Rose and G. Swain reported its synthesis in 1956.
Chlorhexidine is a disinfectant that is added to soaps, cosmetics, toothpaste, and similar products. Veterinarians use it as a topical disinfectant for animals’ wounds. It is active against Gram-positive and Gram–negative bacteria, but not viruses. It is included in the World Health Organization's List of Essential Medicines.
Recently, S. F. Bossmann and co-workers at Kansas State University found a way to use chlorhexidine as an internal antibiotic in animals. They “cloaked” it in the bacterium Micrococcus luteus and delivered it to Fusobacterium necrophorum cultures by inserting the microbe into white blood cells. (F. necrophorum is a rumen bacterium that can enter bovine livers, causing inflammation, which retards growth.) The white cells tame the inflammation; they and M. luteus then degrade to release chlorhexidine, which kills the rumen bacteria.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

