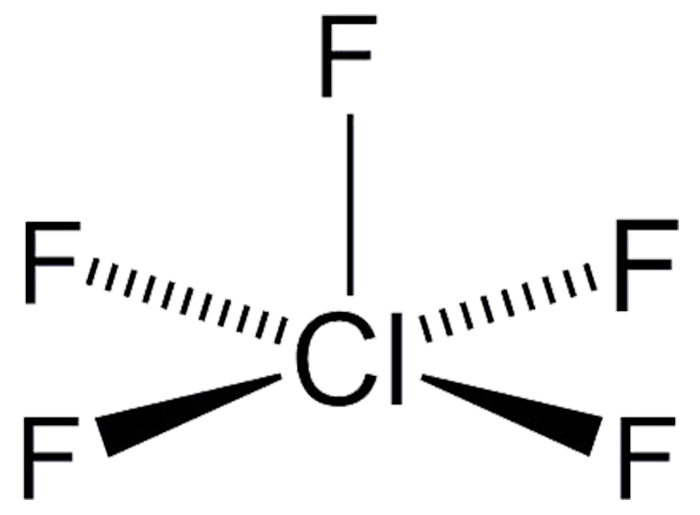
The gas chlorine pentafluoride (ClF5) was first prepared by D. F. Smith in 1963 by the reaction of ClF3 with F2 under heat and pressure. It was previously an unknown compound. Ironically, it was predicted on the basis of the existence of XeF4, which at the time was considered to be a novelty because it was believed that noble gases could not bind to other elements. ClF5 has occasionally been tried as an oxidant, but the HCl and HF byproducts make this a bad idea.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

