What molecule am I?

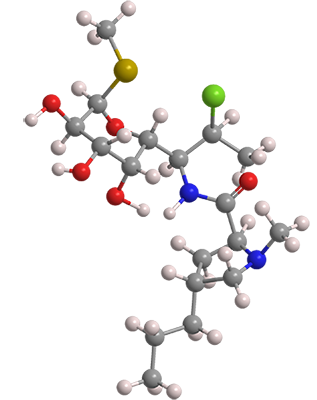
Clindamycin is an older antibiotic that is used to treat bacterial infections including pneumonia, strep throat, osteomyelitis, and endocarditis. It was first synthesized in 1966 by Barney J. Magerlein*, Robert D. Birkenmeyer, and Fred Kagan* at Upjohn (Kalamazoo, MI) by chemically modifying lincomycin1, a natural antibiotic. In the next few years, the same authors published additional articles and patents on clindamycin.
Clindamycin works by inhibiting ribosomal translocation in bacteria, reducing their ability to make proteins. It is most effective against aerobic Gram-positive and some anaerobic Gram-negative bacteria. It was approved by the US Food and Drug Administration in 1970.
Over the years, clindamycin has been a useful, versatile drug; but it is not without side effects such as nausea, diarrhea, and rashes. Significantly, it increases the risk of hospital-acquired colitis caused by Clostridium difficile. Although it has some ability to combat methicillin-resistant Staphylococcus aureus (MRSA), many bacteria have developed resistance to it.
Currently, clindamycin is taking on a new use: the development of more effective antibiotics. For an example, see this month’s Molecule of the Future, below.
1. CAS Reg. No. 154-21-2.
Clindamycin hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—May be harmful if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Skin sensitization | H317—May cause an allergic skin reaction | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Reproductive toxicity, effects on or via lactation*** | H362—May cause harm to breast-fed children | |
| Short-term (acute) aquatic hazard, category 1 | H400—Very toxic to aquatic life | |
| Long-term (chronic) aquatic hazard, category 1 | H410—Very toxic to aquatic life with long-lasting effects | |
*Compilation of multiple safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
***No category or pictogram assigned.
Molecule of the Future
Iboxamycin1, a member of the oxepanoprolinamide class of antibiotics, is under development as a next-generation successor to clindamycin, described above. In July 2021, Andrew G. Myers and colleagues at Harvard University (Cambridge, MA) reported a practical synthesis of iboxamycin in which they replaced the n-propyl group in clindamycin with an oxepane ring fused to the pyrrolidine ring.
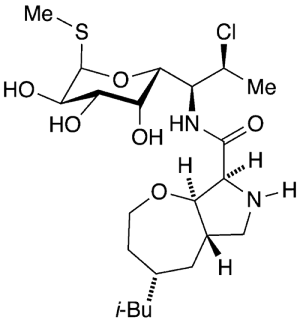
Later that year, Myers and his group, along with Yury. S. Polikanov and co-workers at the University of Illinois at Chicago, expanded on the original work. They reported that iboxamycin overcomes bacteria that are resistant to clindamycin, including strains of Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii. It also expands the range of Gram-positive and Gram-negative bacteria controlled by clindamycin.
Iboxamycin binds to the same place on the bacterial ribosome as does clindamycin, but it defeats the mechanism that many bacteria developed to become resistant to clindamycin.
1. As of this writing, no CAS Reg. No. has been assigned to iboxamycin.
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
Clindamycin fast facts
| CAS Reg. No. | 18323-44-9 |
| SciFinder nomenclature | L-threo-α-D-galacto-Octopyranoside, |
| Empirical formula | C18H33ClN2O5S |
| Molar mass | 424.98 g/mol |
| Appearance | Yellow amorphous solid |
| Melting point | 141–143 °Ca |
| Water solubility | 31 mg/L (est.) |
a. Value for clindamycin hydrochloride, CAS Reg. No. 21462-39-5.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

