
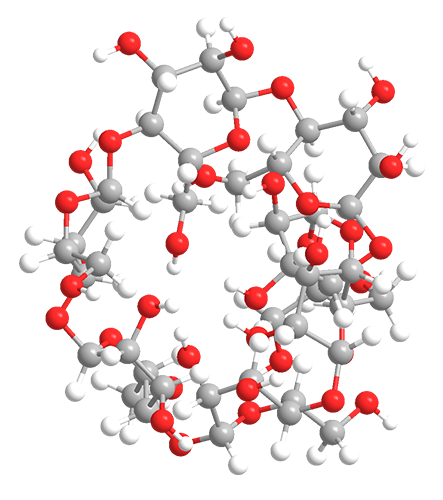
Cyclodextrins are sugar molecules bound together in rings of various sizes. Specifically, the sugar units are called glucopyranosides—glucose molecules that exist in the pyranose (six-membered) ring configuration. Six, seven, or eight glucopyranosides bind with each other to form α-, β-, and γ-cyclodextrin, respectively. β-Cyclodextrin is shown in the images.
The three cyclodextrins occur naturally; they were first described in 1891 by A. Villiers, who called them “cellulosine”. A few years later, A. Schardinger characterized the three variants.
As complex as they are, cyclodextrins are relatively easy to make. Starch is treated with a combination of common enzymes, notably cyclodextrin glycosyl transferase and α-amylase. Each enzyme combination produces a characteristic ratio of the three cyclodextrins.
Cyclodextrins form a toroid (truncated cone) configuration with multiple hydroxyl groups at each end. This allows them to encapsulate hydrophobic compounds without losing their solubility in water.
Among other applications, cyclodextrins can be used to carry hydrophobic drug molecules into biological systems. Cyclodextrins used in the laundry product Febreze trap odor molecules so that they do not reach the scent receptors in your nose.
MOTW update:
April 22, 2019
Cyclodextrins consist of sugar molecules (glucopyranosides) arranged in rings. The 6-, 7-, and 8-membered cyclodextrins occur naturally; and until recently the smallest ring to be synthesized contained five glucopyranoside units. This record was broken earlier this month when Hidetoshi Yamada and co-workers at Kwansei Gakuin University (Nishinomiya, Japan) announced that they made cyclodextrins with three and four sugar units—the smallest possible members of the family.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

