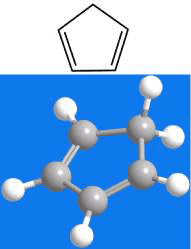
Cyclopentadiene is a liquid with a low boiling point. It cannot be stored at room temperature because it spontaneously undergoes a Diels–Alder reaction with itself that forms the bridged dimer dicyclopentadiene. This reaction is reversible at elevated temperatures, so the most convenient way to obtain cyclopentadiene is to distill the commercially available dimer. Cyclopentadiene reacts with strong bases to form the cyclopentadienyl anion, which acts as a ligand in ferrocene and other “sandwich” complexes.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

