What molecule am I?

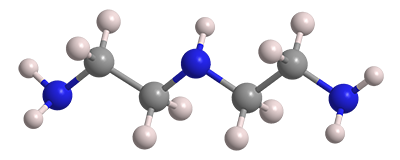
The three amine groups in diethylenetriamine (DETA) make it water-soluble and basic enough to extract acids from gases and hydrocarbon mixtures. Like most organic amines, it has an ammonia-like odor. Its profound toxicity is illustrated by all the category 1 ratings shown in the hazard information table.
The three nitrogen atoms make DETA an excellent complexing agent for metals such as cobalt, copper, and platinum. Some DETA-based metal complexes can be used to catalyze organic reactions; examples are the oxidation of phenol with hydrogen peroxide and Heck cross-coupling reactions.
Recently, Julien Leclaire at the University of Lyon (France) and colleagues there and at the University of Turin (Italy) showed that the cost of carbon dioxide capture with DETA can be offset by another environmentally beneficial process. The chemists devised a way to selectively remove metal contaminants from water by adding DETA to the aqueous solutions, then bubbling CO2 through the mixture. A series of ligand exchange reactions results in the formation of an insoluble carbonate of the target metal.
The authors demonstrated their technique by removing lanthanum as La2(CO3)3 from a La–Ni mixture and by separating the solubilized metals from a La–Co–Ni battery anode alloy into the individual components with 97% or greater purity. Their aim is to collaborate with metal recyclers to develop and commercialize their technology.
Diethylenetriamine hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Acute toxicity, dermal, category 4 | H312—Harmful in contact with skin | |
| Skin corrosion/irritation, category 1B | H314—Causes severe skin burns and eye damage | |
| Skin sensitization, category 1 | H317—May cause an allergic skin reaction | |
| Serious eye damage, category 1 | H318—Causes serious eye damage | |
| Acute toxicity, inhalation, category 1 | H330—Fatal if inhaled | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 1 | H335—May cause respiratory irritation | |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
Diethylenetriamine
fast facts
| CAS Reg. No. | 111-40-0 |
| SciFinder nomenclature | 1,2-Ethanediamine, N-(2-aminoethyl)- |
| Empirical formula | C4H13N3 |
| Molar mass | 103.17 g/mol |
| Appearance | Colorless to yellow liquid |
| Boiling point | 207 ºC |
| Water solubility | Miscible |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

