What molecule am I?

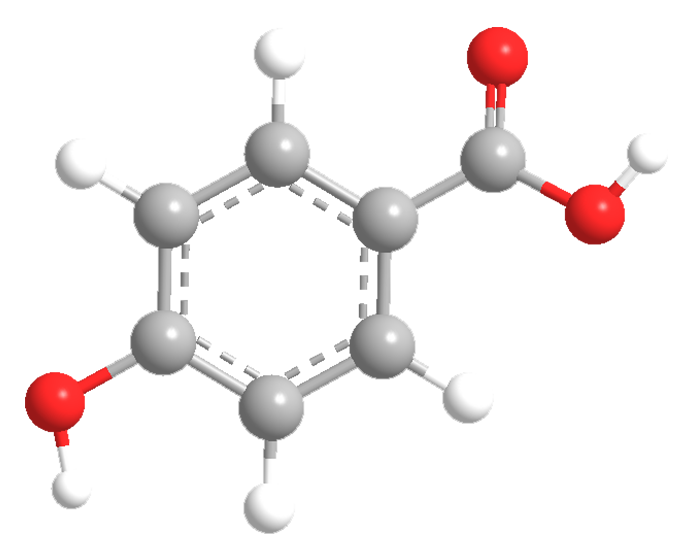
4-Hydroxybenzoic acid (4-HBA) is a white crystalline solid that is widely used in organic synthesis. In particular, its esters, called parabens, are used as preservatives in cosmetics and pharmaceuticals.
In 1947, H. Gilman and C. E. Arntzen at Iowa State College (Ames) reported the synthesis of 4-HBA from 4-hydroxybenzaldehyde. Several other preparation methods were developed since then. Today it is produced commercially via the Kolbe–Schmitt reaction, which originated in the mid-1800s. Potassium phenoxide and CO2 are heated under pressure; the reaction mixture is then acidified to obtain the product. (Oddly, if the sodium salt is used, the product is 2-hydroxybenzoic acid, or salicylic acid.)
Recently, a new role for 4-HBA was discovered. Douglas Rasher*, Mark Hay, and colleagues at Georgia Tech (Atlanta) found that the sea slug Elysia tuca locates its food source, the seaweed Halimeda incrassate, by sensing 4-HBA and halimedatetraacetate, which the plant produces. The slug then pierces the seaweed to obtain chloroplasts, photoreceptors that help the invertebrate convert sunlight into energy.
*Now at the University of Maine (Walpole).

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

