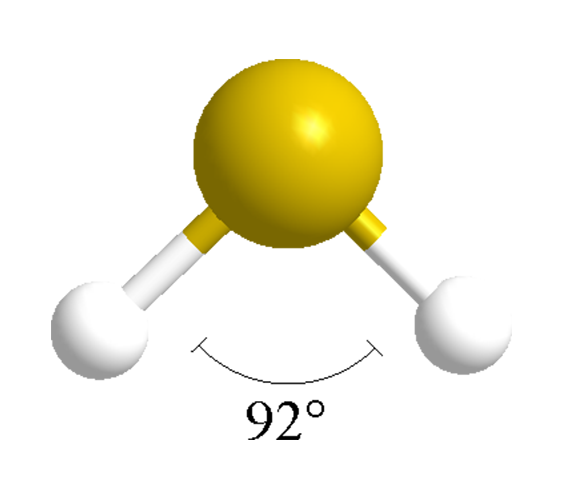
Hydrogen sulfide (H2S) is a dense, colorless gas. At low concentrations, it has a characteristic “rotten egg” odor; at high concentrations, it is extremely toxic and even explosive. It is abundant in nature, mainly as a result of the anaerobic decay of sulfur-containing organic matter. It is also produced in large quantities in oil refineries from the desulfurization of petroleum fractions.
Credit for the discovery of H2S is usually attributed to Swedish chemist C. W. Scheele in 1777. Because the byproduct H2S is made in large quantities, there is little need to manufacture it “on purpose”. Almost all refinery H2S is reduced to nontoxic elemental sulfur via the venerable Claus process, invented by German chemist. C. F. Claus in 1883.
H2S has the formula of an inorganic acid; but in aqueous solution, the dissociation constant (Ka) for the first proton is only ≈1 x 10–7. The second proton’s Ka is a miniscule 1 x 10–12. H2S precipitates many inorganic cations [e.g., Cu(II), Pb(II), and Hg(II)], making it useful for the qualitative detection of these ions.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

