
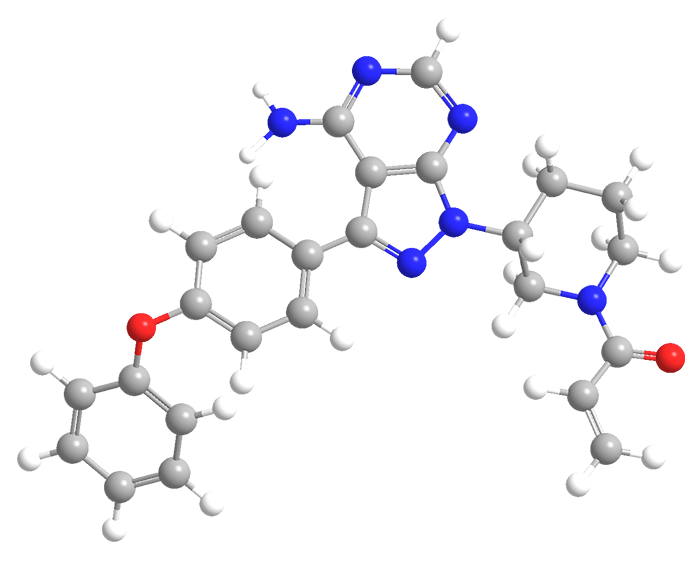
Ibrutinib is a cancer drug that targets B-cell malignancies such as certain leukemias and lymphomas. Its design and synthesis were reported in 2007 by Z. Pan and co-workers at Celera Genomics (South San Francisco, CA, and Rockville, MD). By that time, Pharmacyclics (Sunnyvale, CA) had acquired ibrutinib and related compounds.
Ibrutinib was approved by the US Food and Drug Administration in 2013 for treating mantle cell lymphoma and in 2014 for treating chronic lymphocytic leukemia. The lymphoma application was submitted in June 2013 and, under FDA’s breakthrough therapy program, it was approved 4 months later.
The 2013 approval was also noteworthy because Pharmacyclics had sufficient quantities of the drug to make it commercially available immediately. A key factor was Pharmacyclics’ long partnership with Lonza, a Switzerland-based multinational company with custom manufacturing facilities in the United States.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

