What molecule am I?
Iridium, atomic number 77, is a metallic element in the period 6 transition group. It is quite rare and has relatively few compounds, natural or synthetic. Despite its scarcity, it can exist in oxidation states ranging from –3 to +9.
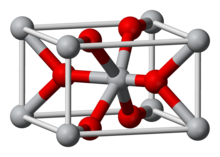
The most common oxide of iridium by far has the +4 oxidation state: iridium(IV) oxide, or IrO2. Ir2O3 [Ir(III)] and IrO4 [Ir(VIII)] are also known. As the image shows, crystalline IrO2 adopts the rutile (TiO2) structure with iridium coordinated to six oxygen atoms.
IrO2 is prepared by heating elemental iridium or IrCl3 in the presence of oxygen. Additional heating causes the oxide to decompose into its elements. Most current IrO2 applications involve its deposition on substrates such as carbon nanotubes.
IrO2 has beneficial properties such as inertness under biological conditions and usefulness in catalysis and electrical devices. Its applications include electrodes for industrial electrolysis and regenerative fuel cells and materials for minimally invasive devices such as stents for treating coronary heart disease.
Iridium(IV) oxide hazard information
| GHS classification*: oxidizing solids, category 3 | |
| H272—May intensify fire; oxidizer | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Iridium(IV) oxide fast facts
| CAS Reg. No. | 12030-49-8 |
| Empirical formula | IrO2 |
| Molar mass | 224.22 g/mol |
| Appearance | Blue or black crystals or powder |
| Melting point | 1100 ºC dec. |
| Water solubility | 2 mg/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

