What molecule am I?
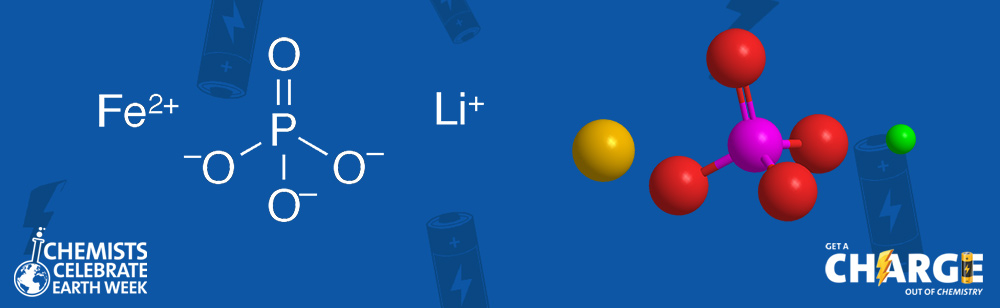
Lithium iron phosphate (LiFePO4) is a compound salt with an olivine (LiMPO4) structure that has a particular application in battery cathodes. The substance was first reported in the chemical literature by Ralph P. Santoro and Robert E. Newnham at MIT (Cambridge, MA) in a 1966 US Air Force Materials Laboratory survey of magnetoelectric materials. The following year, the same authors described the magnetic structure of LiFePO4, which showed that it is antiferromagnetic.
In a 2009 article, Dragana Jugović* and Dragan Uskoković at the Institute of Technical Sciences of the Serbian Academy of Sciences and Arts (Belgrade) reviewed procedures for preparing LiFePO4 powders for use in batteries. The compound occurs in nature as the mineral triphylite1, but that source lacks the purity required for battery applications.
Hundreds of technical articles have explored the use of LiFePO4 in cathodes. In an example this month, Yitao He*, Xiaoxiang Shen, and Yaohui Zhang* at Anhui University of Technology (Ma’anshan) and Harbin Institute of Technology (both in China) reviewed the use of nanosized LiFePO4 and other cathode salts in layered 2-D batteries. The authors explained how the nanomaterials enhance battery performance.
The recycling and reuse of battery materials is highly desirable. Also this month, Lei Song, Hairong Yue, and co-workers at Sichuan University (Chengdu, China) proposed a two-step process for recycling LiFePO4 in spent cathodes:
- In an acid-free system, lithium is leached from the cathode using potassium persulfate2 (K2S2O8) as the leaching agent. K2S2O8 also oxidizes the iron to iron(III) phosphate3 (FePO4).
- The residual carbon4 in the leaching residue is used to reduce FePO4 to iron(II) pyrophosphate5 (Fe2P2O7), which is a component of some battery anodes.
LiFePO4 is used in ≈30% of lithium-ion batteries in electric vehicles, increasing the demand for the compound. In September 2023, LG Chem (Seoul, South Korea) and Huayou Group (Tongxian, China) announced a plan to build a LiFePO4 cathode materials plant in Morocco. The plant is expected to produce enough material for 500,000 EV batteries annually when it starts up in 2026.
This Earth Week, join your fellow chemists to celebrate batteries!
1. CAS Reg. No. 13816-45-0.
2. CAS Reg. No. 7727-21-1.
3. CAS Reg. No. 10045-86-0.
4. The carbon sources are the poly(vinylidene fluoride) binder and the conductive carbon in the cathode.
5. CAS Reg. No. 15600-46-1.
Lithium iron phosphate hazard information
| Hazard class* | GHS code and hazard statement |
|---|---|
| Not a hazardous substance or mixture |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.
MOTW update
Acetylene1 was the Molecule of the Week for January 15, 2007. It is the simplest molecule that contains a carbon–carbon triple bond. Its main use is as a fuel for welding and cutting metals; it has also served as a reactant for synthesizing acetaldehyde and acetic acid.
Acetylene is rarely found on Earth, but it is surprisingly abundant in space. It exists on icy planets and moons, the cold interstellar medium, and extremely hot circumstellar surroundings. This month, a review by Evgeniy O. Pentsak, Maria S. Murga, and Valentine P. Ananikov* at the Russian Academy of Sciences (Moscow) describes how extraterrestrial acetylene forms and expands carbon skeletons, including aromatic rings and even nanosized particles, in cold and hot environments. The authors also explore acetylene’s role in prebiotic chemistry.
1. CAS Reg. No. 74-86-2.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Lithium iron phosphate fast facts
| CAS Reg. No. | 15365-14-7 |
| SciFindern name | Phosphoric acid, iron(2+) lithium salt (1:1:1) |
| Empirical formula | FeLiO4P |
| Molar mass | 157.76 g/mol |
| Appearance | Gray, brown, or black crystals or powder |
| Melting point | >300 °C |
| Water solubility | Insoluble |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

