What molecule am I?

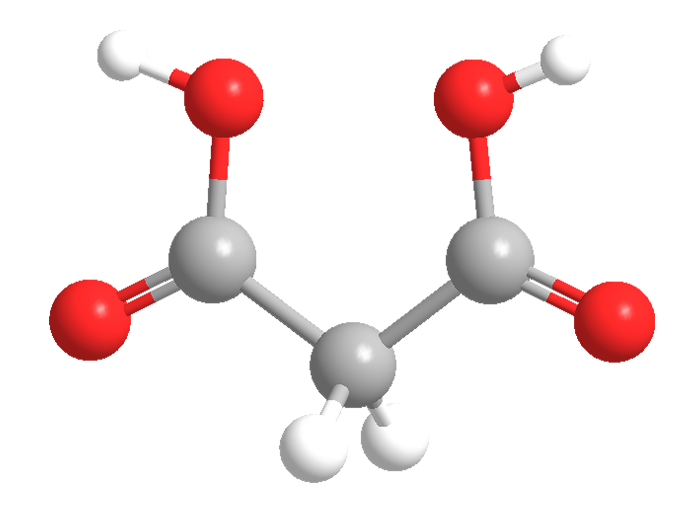
Malonic acid, formally propanedioic acid, is the second-smallest aliphatic dicarboxylic acid. (Oxalic acid is the smallest.) It should not be confused with malic or maleic acid, both of which also contain two carboxyls.
Malonic acid is a white crystalline solid with a decomposition point of ≈135 °C. It is highly soluble in water and oxygenated solvents. It has numerous commercial uses: It is a precursor to specialty polyesters; it is used in the manufacture of barbiturates, coatings, and biodegradable containers; and it is even a component of surgical adhesives.
French chemist Victor Dessaignes reported the first synthesis of malonic acid in 1858; he made it by oxidatively decomposing four-carbon malic acid with potassium dichromate. Since then, it has been synthesized commercially starting from chloroacetic acid, diethyl malonate, and even sodium acetate.
In the past two decades, much work has been done on biobased syntheses of malonic acid. Most recently, the Berkeley, CA, venture-capital startup Lygos raised US$13 million to scale up a process that uses bioengineered yeast strains to produce malonic acid from sugars.
Lygos, by the way, was reported by Pliny the Elder as a former name of the “town of Byzantium”. Today, of course, it’s the city of Istanbul.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.


