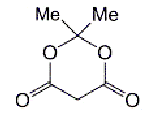
In 1908, A. N. Meldrum condensed malonic acid with acetone to produce a compound he misidentified as a carboxylic acid—hence the name “Meldrum’s acid”. Not until 1948 did D. Davidson and S. A. Bernhard show that the compound has the dioxane-dione structure shown here. Its acidity derives from the lability of the hydrogen atoms between the carbonyl groups. The same authors noted that Meldrum’s acid is a ketene precursor. Recently B. Moon and C. J. Hawker used this property to create polymers with reactive ketene substituents.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

