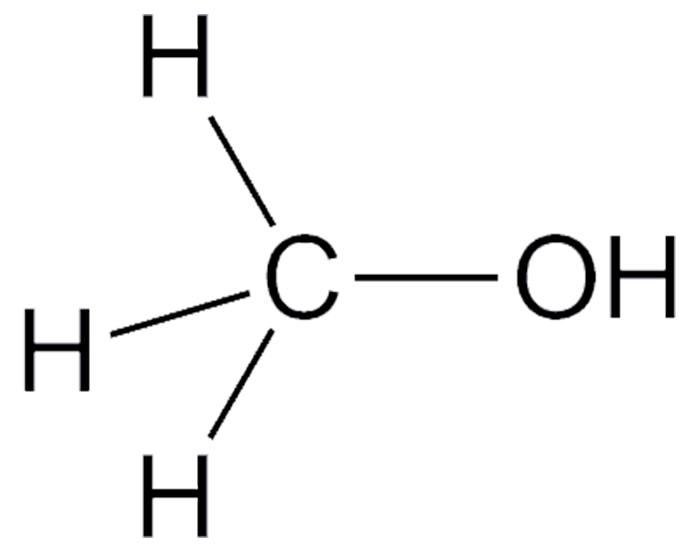
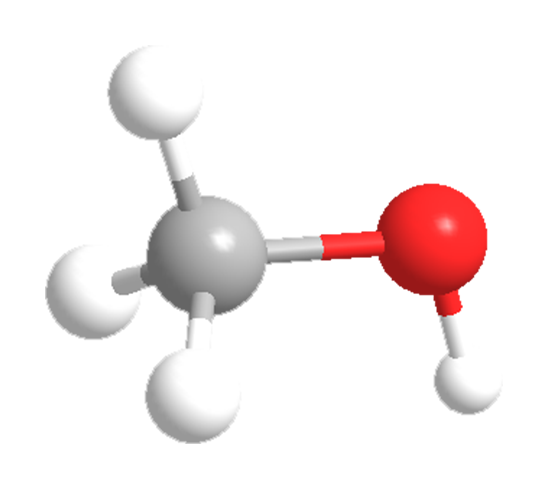
Methanol (MeOH), the simplest alcohol, is widely used as a solvent, motor fuel, ethanol denaturant, and, most of all, a feedstock for manufacturing other chemicals. It was originally made by the destructive distillation of wood—hence, the once commonly used name “wood alcohol”.
Methanol is miscible with water and with almost every other organic solvent. It is colorless, volatile, flammable, and poisonous. During Prohibition, many people died from drinking methanol-laced liquor.
Methanol is an old chemical, but it still makes news. K. Rajeshwar and colleagues at the University of Texas, Arlington, recently developed a process that uses solar power to make methanol from CO2 .

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

